Question
The table below shows rate constants of ligand substitution reactions with various entering ligands for [Cr(NH3)5(HO)]+ and [Cr(HO)]+. Rate Constants for Anation Entering Ligand
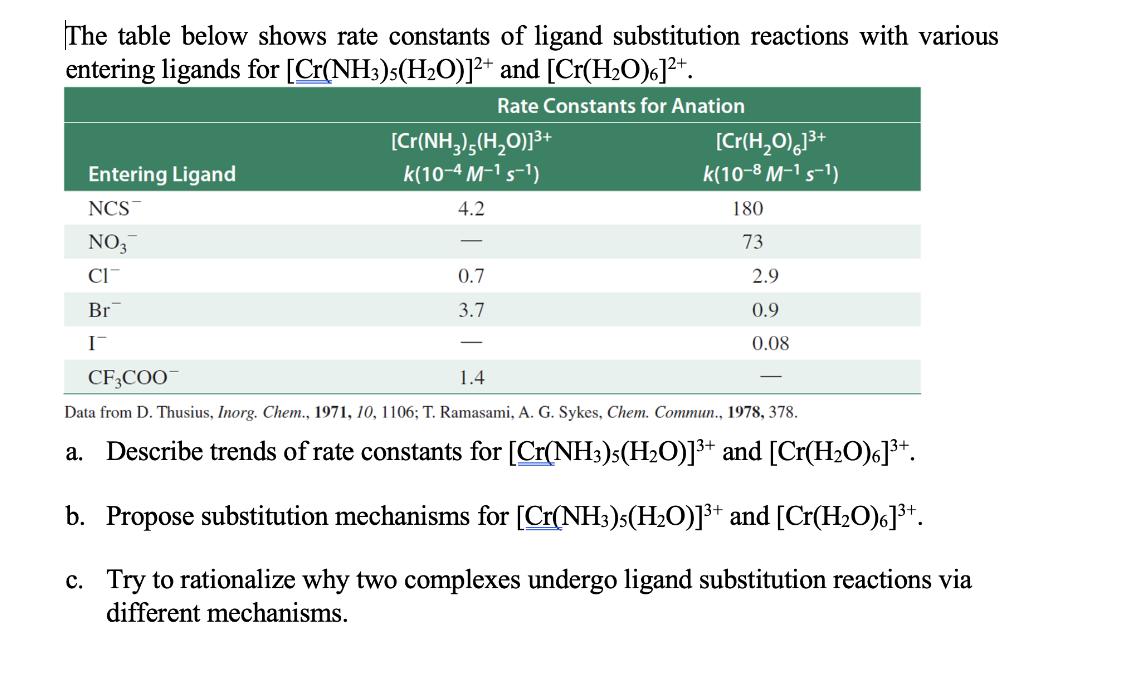
The table below shows rate constants of ligand substitution reactions with various entering ligands for [Cr(NH3)5(HO)]+ and [Cr(HO)]+. Rate Constants for Anation Entering Ligand NCS NO3 CIT Br I [Cr(NH3)5(HO)]+ k(10-4 M- S-) 4.2 - 0.7 3.7 [Cr(HO)]+ k(10-8 M- S-) 180 73 2.9 0.9 0.08 CF3COO 1.4 Data from D. Thusius, Inorg. Chem., 1971, 10, 1106; T. Ramasami, A. G. Sykes, Chem. Commun., 1978, 378. a. Describe trends of rate constants for [Cr(NH3)5(HO)]+ and [Cr(HO)6]+. b. Propose substitution mechanisms for [Cr(NH3)5(HO)]+ and [Cr(HO)]+. c. Try to rationalize why two complexes undergo ligand substitution reactions via different mechanisms.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



