Answered step by step
Verified Expert Solution
Question
1 Approved Answer
At 10. atm and 100 K, radon (Rn) deviates from its predicted volume based on the ideal gas law if sen has a smaller
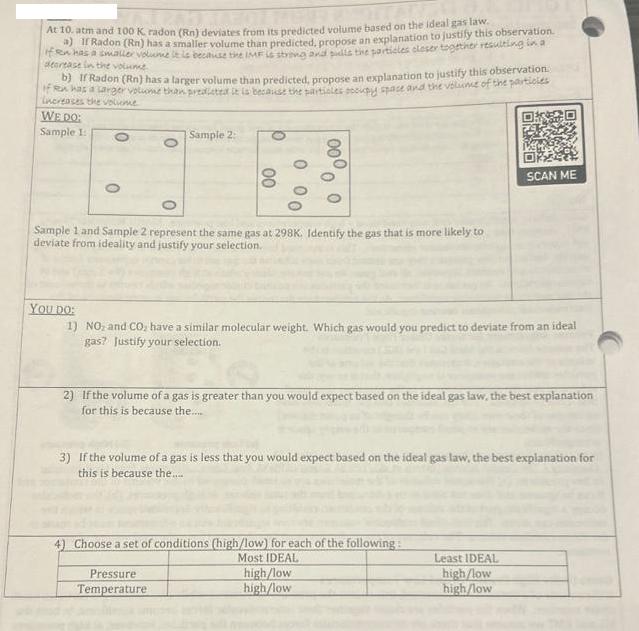
At 10. atm and 100 K, radon (Rn) deviates from its predicted volume based on the ideal gas law if sen has a smaller volume it is because the IMF is strong and pulls the particles closer together resulting in a smaller volume than predicted, propose an explanation to justify this observation. Radon (Rn) has a decrease in the volume b) If Radon (Rn) has a larger volume than predicted, propose an explanation to justify this observation. If en has a larger volume than prealeted it is because the particles occupy space and the volume of the particles Increases the voliome WE DO: Sample 1: O 0 0 Sample 2: 00 0 000 00 0 Sample 1 and Sample 2 represent the same gas at 298K. Identify the gas that is more likely to deviate from ideality and justify your selection. Pressure Temperature YOU DO: 1) NO and CO, have a similar molecular weight. Which gas would you predict to deviate from an ideal gas? Justify your selection. 2) If the volume of a gas is greater than you would expect based on the ideal gas law, the best explanation for this is because the...... 3) If the volume of a gas is less that you would expect based on the ideal gas law, the best explanation for this is because the.... 4) Choose a set of conditions (high/low) for each of the following: Most IDEAL SCAN ME high/low high/low Least IDEAL high/low high/low
Step by Step Solution
★★★★★
3.42 Rating (168 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


