Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What is the answer to the question mark? please show step by step solutions. thanks. 100 tons of liquid steel (at 1600C ) has been
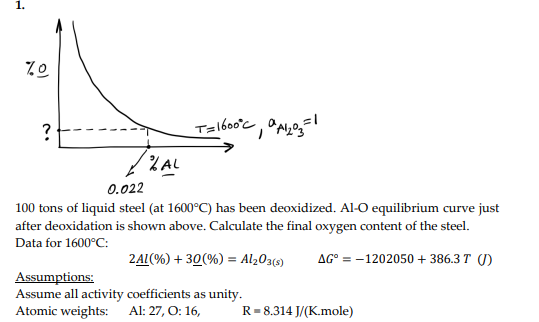
What is the answer to the question mark? please show step by step solutions. thanks.
100 tons of liquid steel (at 1600C ) has been deoxidized. Al-O equilibrium curve just after deoxidation is shown above. Calculate the final oxygen content of the steel. Data for 1600C : 2Al(%)+3O(%)=Al2O3(s)G=1202050+386.3T(J) Assumptions: Assume all activity coefficients as unity. Atomic weights: Al: 27, O: 16, R=8.314J/(K.mole)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


