Question
What is the approximate pka for the weak acid. pH 14 12- 10- 8 6 4 2 0 0 Answer: 2 (c) Equivalence point
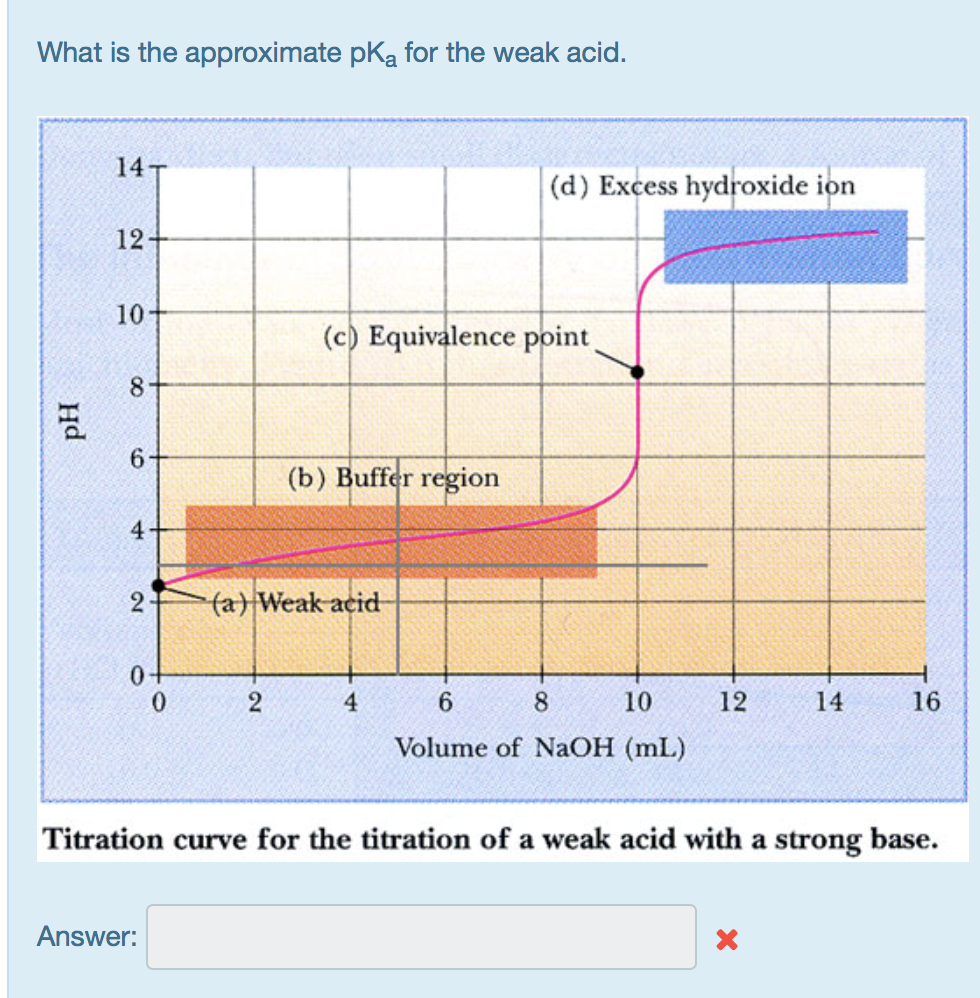
What is the approximate pka for the weak acid. pH 14 12- 10- 8 6 4 2 0 0 Answer: 2 (c) Equivalence point (b) Buffer region (a) Weak acid (d) Excess hydroxide ion 4 6 8 10 Volume of NaOH (mL) 12 14 Titration curve for the titration of a weak acid with a strong base. X 16
Step by Step Solution
3.49 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
pKa is the equal to pH at half equivalen...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Precalculus
Authors: Jay Abramson
1st Edition
1938168348, 978-1938168345
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



