Question
A 26.6 g sample of Cu metal (c = 0.39 J/g C) is heated to 87.0C and then placed into a beaker containing 181
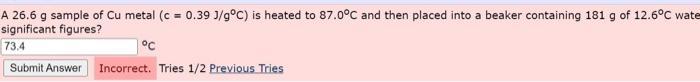
A 26.6 g sample of Cu metal (c = 0.39 J/g C) is heated to 87.0C and then placed into a beaker containing 181 g of 12.6C wate significant figures? 73.4 Submit Answer Incorrect. Tries 1/2 Previous Tries
Step by Step Solution
3.28 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
ANSWER The final temperature of the Cu metal and water under give...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Forensic Accounting and Fraud Examination
Authors: Mary Jo Kranacher, Richard Riley, Joseph T. Wells
1st edition
047043774X, 978-0470437742
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



