Question
What is the empirical formula of a compound that is 62.0 % C, 10.4% H, and 27.5% O by mass? Fill in the blanks
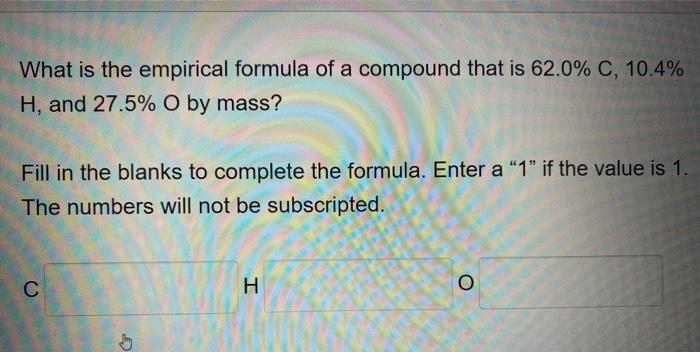
What is the empirical formula of a compound that is 62.0 % C, 10.4% H, and 27.5% O by mass? Fill in the blanks to complete the formula. Enter a "1" if the value is 1. The numbers will not be subscripted. C G H O
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Compositron by mas Molar Mass No of moles ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



