Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What is the order for iodone? Calculated Data Part A: 1981s 99.95 Reaction Molarity of Molarity Molarity of Average Reaction Rate of Reaction Mixture Acetone
What is the order for iodone? 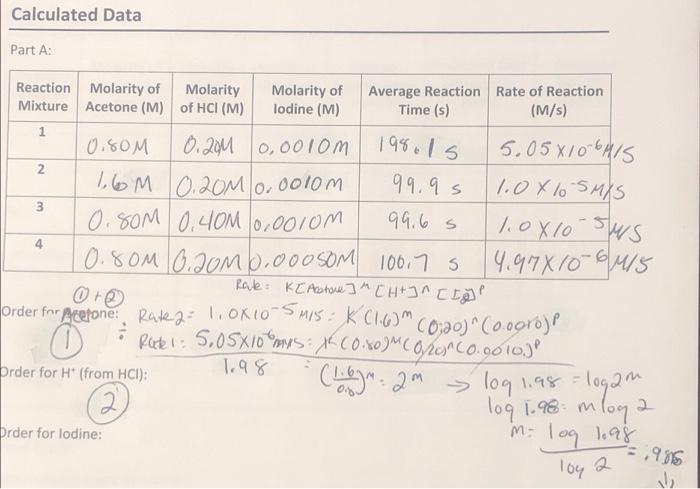
Calculated Data Part A: 1981s 99.95 Reaction Molarity of Molarity Molarity of Average Reaction Rate of Reaction Mixture Acetone (M) of HCI (M) lodine (M) Time (s) (M/s) 1 0,80M 0.20M 0,00lom 5.05X/06H15 2 16M 0.20M 0.0010m 1.0X lo smis 3 0.80M 0.40M 0,0010m 99.6 s 10x10-sus 0.80M 0.JOM 1,000 SOM 100,75 14.97810-8415 Rate: K[Actore] "CH+J^ [Igi 0 @ Order for Acetone: Rated: loxio-5M15: KC16) "CO;20) "(0.00rb)P 0 Ruti: 5,05X10mys X COX0M Coron Co.9010.) 1.98 Order for H" (from HCI): . (1.bg: 2 m = logam log 1.96 mloga Order for lodine: 'm-log 1.98 104 a > log 1,98=1 5,985 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


