Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What is the rate constant at this temperature? The gas-phase decomposition of SO2Cl2. SO2Cl2(g)SO2(g)+Cl2(g), is first order in SO2Cl2.At600K Express your answer using two significant
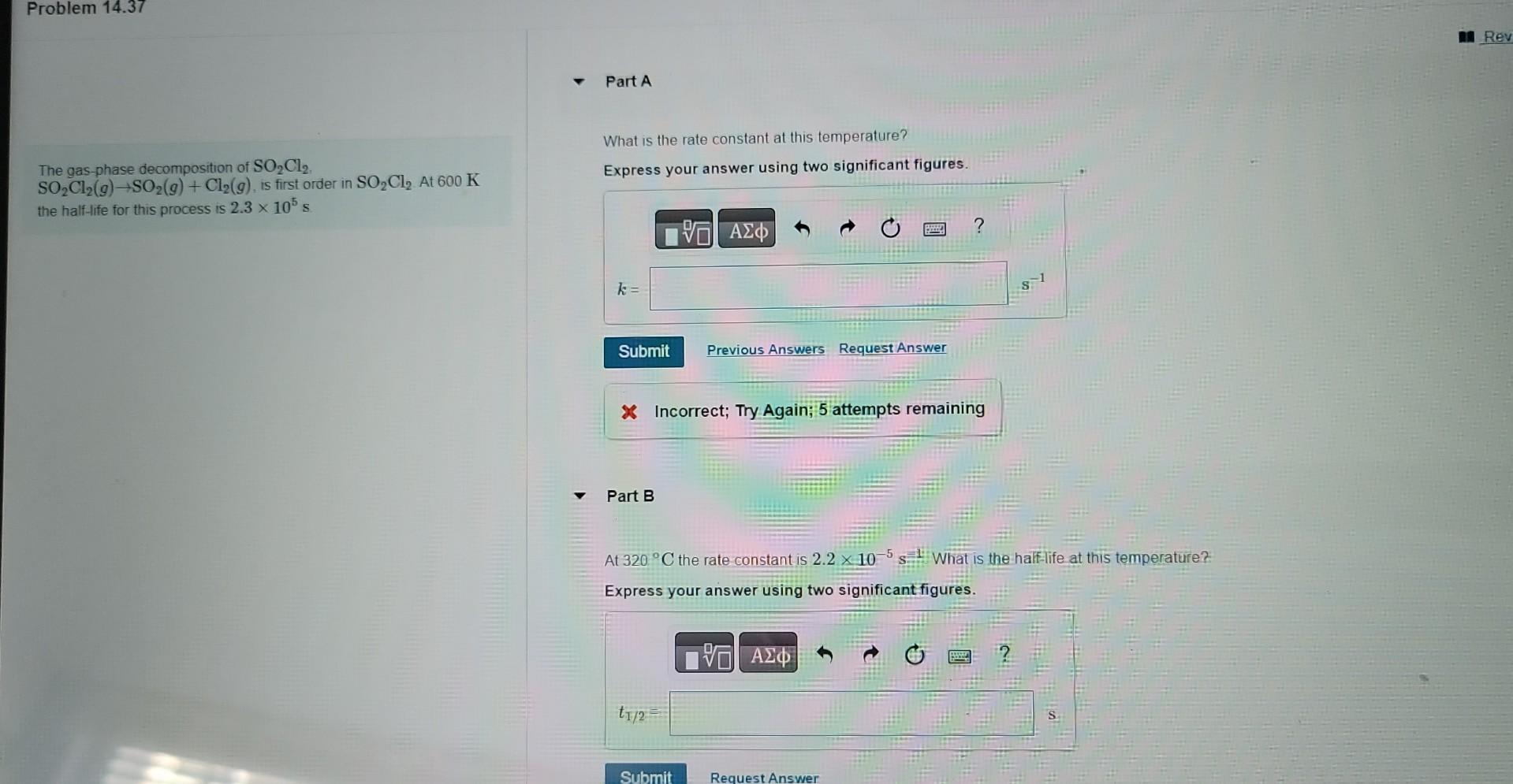
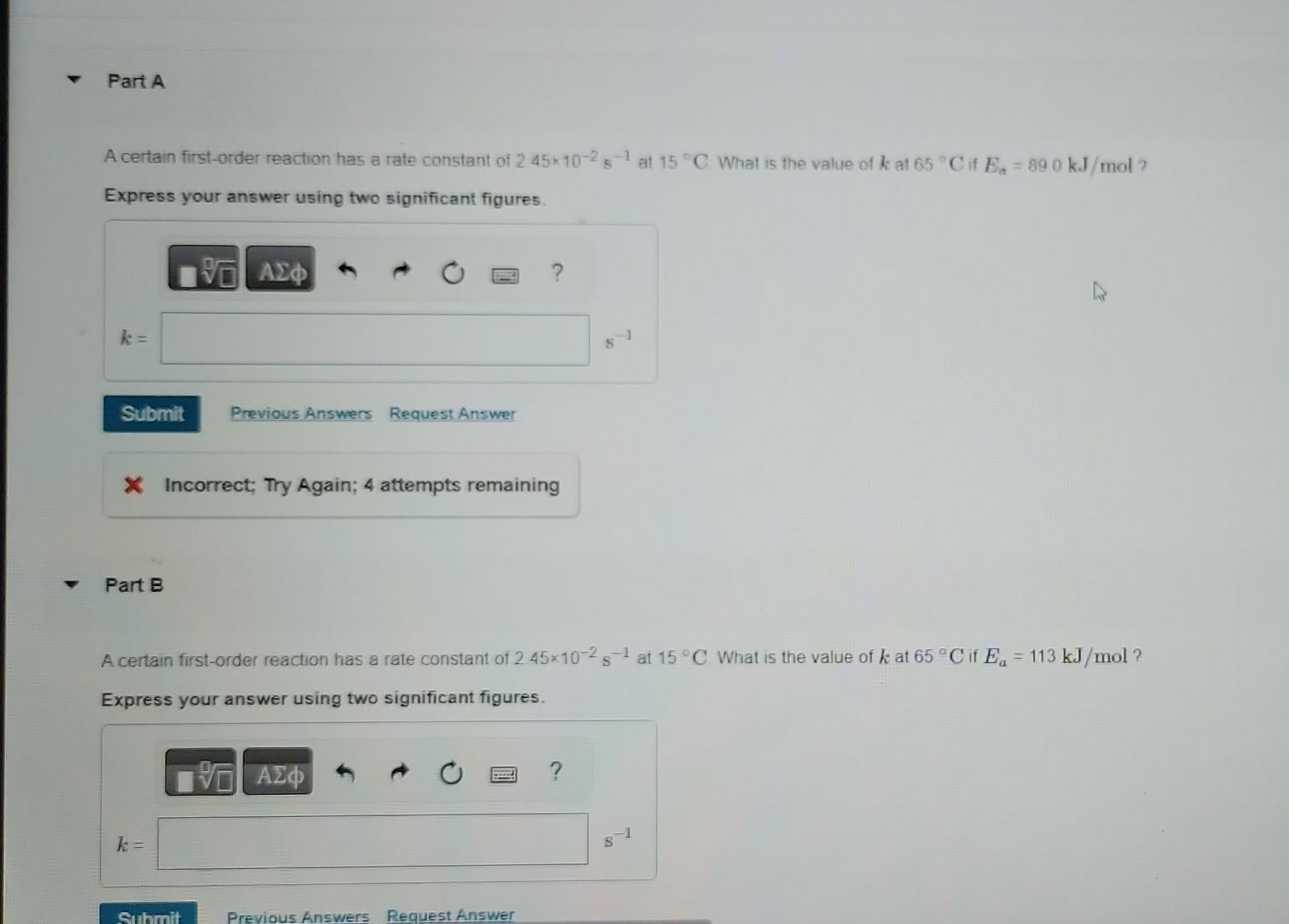
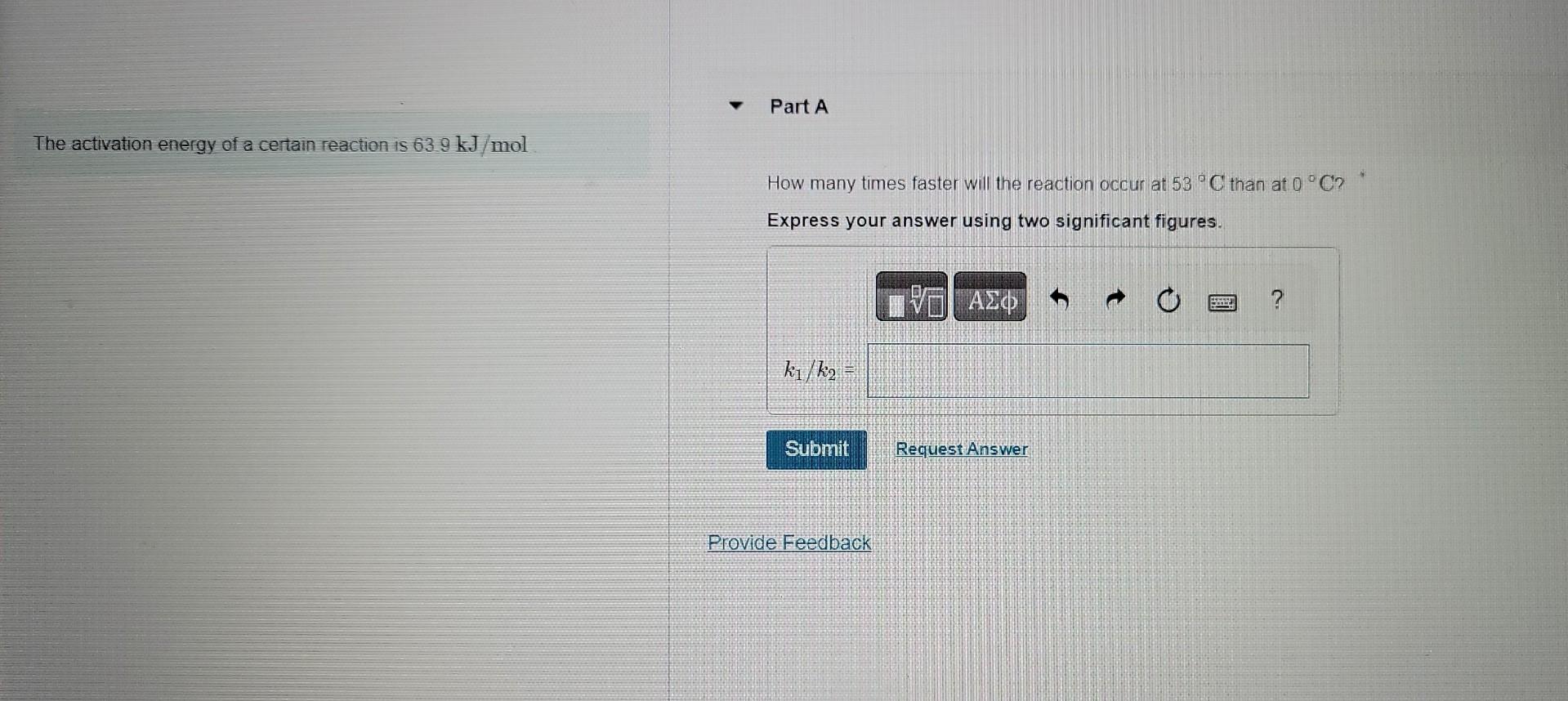
What is the rate constant at this temperature? The gas-phase decomposition of SO2Cl2. SO2Cl2(g)SO2(g)+Cl2(g), is first order in SO2Cl2.At600K Express your answer using two significant figures. the half-life for this process is 2.3105s. X Incorrect; Try Again; 5 attempts remaining Part B At 320C the rate constant is 2.2105s=1 What is the half life at this temperature? Express your answer using two significant figures. A certain first-order reaction has a rate constant of 245102s1 at 15C What is the value of k at 65C if Ea=890kJ/mol ? Express your answer using two significant figures. k= x. Incorrect; Try Again; 4 attempts remaining Part B A certain first-order reaction has a rate constant of 245102s1 at 15C. What is the value of k at 65C if Ea=113kJ/mol ? Express your answer using two significant figures. The activation energy of a certain reaction is 63.9kJ/mol How many times faster will the reaction occur at 53C than at 0C ? Express your answer using two significant figures
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


