What is the rate law for thefollowing mechanism in terms of the overall rate constant k? Step1: A + B ? C (fast) Step 2:
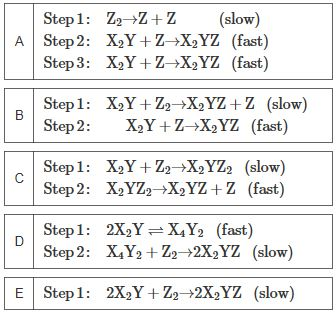 What is the rate law for thefollowing mechanism in terms of the overall rate constant k? Step1: A + B ? C (fast) Step 2: B + C ? D (slow) a) Express your answerin terms of k and the necessary concentrations (e.g., k[A]3[D]). b)Consider the reaction: 2X2Y + Z2 ? 2X2YZ which has a rate law of:rate = k[X2Y][Z2] Select a possible mechanism for the reaction.
What is the rate law for thefollowing mechanism in terms of the overall rate constant k? Step1: A + B ? C (fast) Step 2: B + C ? D (slow) a) Express your answerin terms of k and the necessary concentrations (e.g., k[A]3[D]). b)Consider the reaction: 2X2Y + Z2 ? 2X2YZ which has a rate law of:rate = k[X2Y][Z2] Select a possible mechanism for the reaction.
Step 1: Z Z+Z A Step 2: XY+ZXYZ Step 3: B C Step 1: Step 2: D Step 1: Step 2: E Step 1: (slow) (fast) XY+ZXYZ (fast) XY+Z2 XYZ+Z (slow) XY+ZXYZ (fast) Step 1: XY+Z2 XYZ2 (slow) Step 2: XYZ2 XYZ+Z (fast) 2XYXY (fast) X4Y2+Z2-2X2YZ (slow) 2XY+Z2-2XYZ (slow)
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


