Question
What is the stronger acid in the following reaction if the equilibrium constant is much less than 0.01? HNO3 + H,SO4 H,O3 + HS04
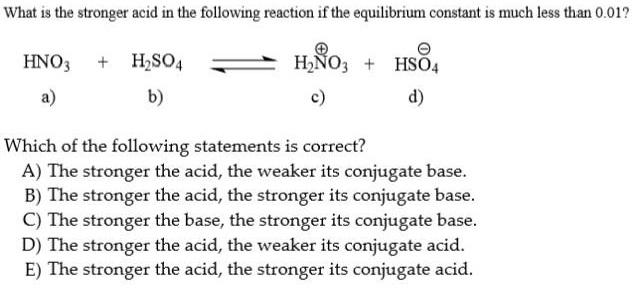
What is the stronger acid in the following reaction if the equilibrium constant is much less than 0.01? HNO3 + H,SO4 H,O3 + HS04 a) b) c) d) Which of the following statements is correct? A) The stronger the acid, the weaker its conjugate base. B) The stronger the acid, the stronger its conjugate base. C) The stronger the base, the stronger its conjugate base. D) The stronger the acid, the weaker its conjugate acid. E) The stronger the acid, the stronger its conjugate acid.
Step by Step Solution
3.45 Rating (145 Votes )
There are 3 Steps involved in it
Step: 1
3 At the given set of equilibrium ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Discrete Mathematics and Its Applications
Authors: Kenneth H. Rosen
7th edition
0073383090, 978-0073383095
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



