Question
What is the theoretical yield in grams for this reaction under the given conditions?Express the answer to three significant figures and include the appropriate units.
What is the theoretical yield in grams for this reaction under the given conditions?Express the answer to three significant figures and include the appropriate units.
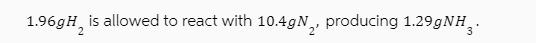
1.96gH2 is allowed to react with 10.4gN, producing 1.29gNH.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Based on the image you sent the theoretical yield for the reaction is 20 grams of NH3 The balanced c...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introductory Chemistry Atoms First
Authors: Steve Russo And Michael Silver
5th Edition
0321927117, 9780321927118
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



