What makes a good leaving group? 4. Some reactions are more sensitive to the identity of the leaving group than others-that is, they require
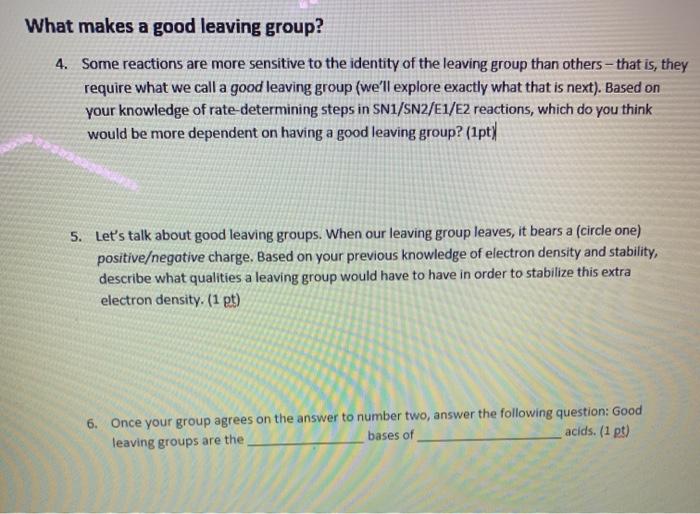
What makes a good leaving group? 4. Some reactions are more sensitive to the identity of the leaving group than others-that is, they require what we call a good leaving group (we'll explore exactly what that is next). Based on your knowledge of rate-determining steps in SN1/SN2/E1/E2 reactions, which do you think would be more dependent on having a good leaving group? (1pt) 5. Let's talk about good leaving groups. When our leaving group leaves, it bears a (circle one) positive/negative charge. Based on your previous knowledge of electron density and stability, describe what qualities a leaving group would have to have in order to stabilize this extra electron density. (1 pt) 6. Once your group agrees on the answer to number two, answer the following question: Good acids. (1 pt) leaving groups are the bases of
Step by Step Solution
3.37 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Ans4 SN1 reaction are the most dependent on the leaving group ...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


