Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What the answer ana why ?! 2 15 mL of 0.25 mol L aqueous silver nitrate reacts with 0.10 g of zinc as follows: AgNO3
What the answer ana why ?! 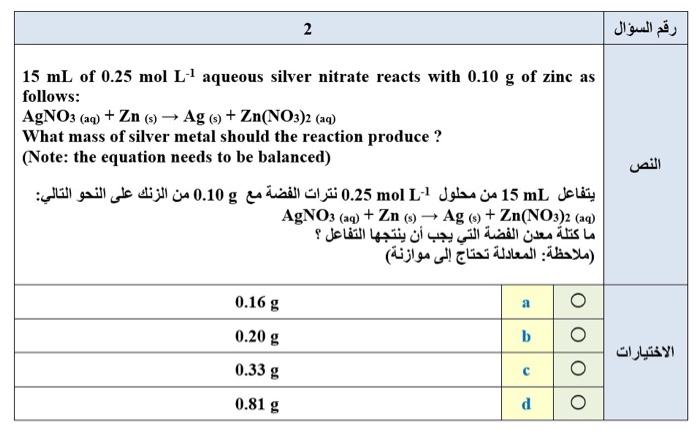
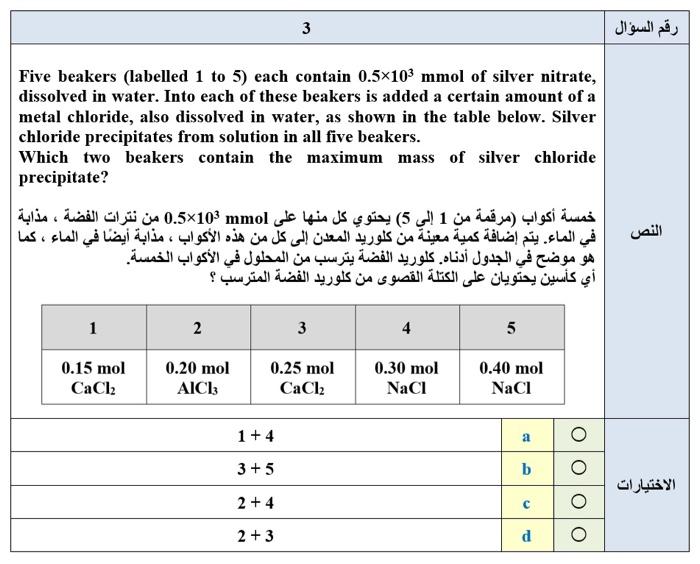
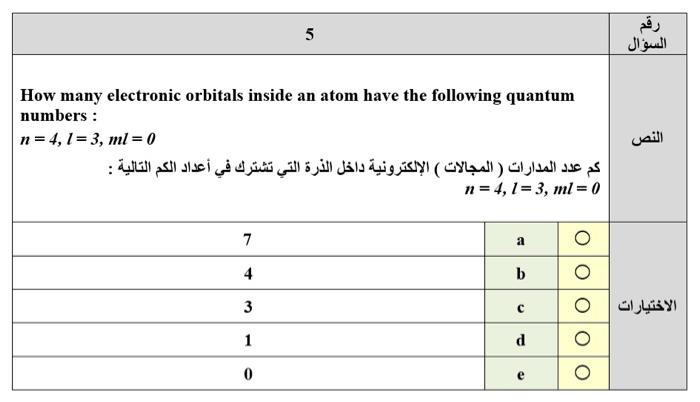
2 15 mL of 0.25 mol L aqueous silver nitrate reacts with 0.10 g of zinc as follows: AgNO3 (aq) + Zn (s) - Ag (s) + Zn(NO3)2 (aq) What mass of silver metal should the reaction produce ? (Note: the equation needs to be balanced) mL 15 mol L1 0.25 g 0.10 : AgNO: (aq) + Zn (s) - Ag (s) + Zn(NO3)2 (aq) : ) 0.16 g 0.20 g b 0.33 g C 0.81g d 3 Five beakers (labelled 1 to 5) each contain 0.5x103 mmol of silver nitrate, dissolved in water. Into each of these beakers is added a certain amount of a metal chloride, also dissolved in water, as shown in the table below. Silver chloride precipitates from solution in all five beakers. Which two beakers contain the maximum mass of silver chloride precipitate? ( 1 5) 0.5x105 mmol | . | . 1 2 3 4 5 0.15 mol CaCl2 0.20 mol AICI: 0.25 mol CaCl2 0.30 mol NaCl 0.40 mol NaCI 1 + 4 a 3 + 5 b 2 + 4 C 2 + 3 d O 5 How many electronic orbitals inside an atom have the following quantum numbers : | n = 4,1 = 3, ml = 0 ( ) : n = 4, 1= 3, ml = 0 7 0 4 b 3 C 1 d 0 e 0 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


