Answered step by step
Verified Expert Solution
Question
1 Approved Answer
what the answer Pure chlorobenzene is contained in a flask attached to an open-end mercury manometer When the flask coritents are at 58.39C, the height
what the answer 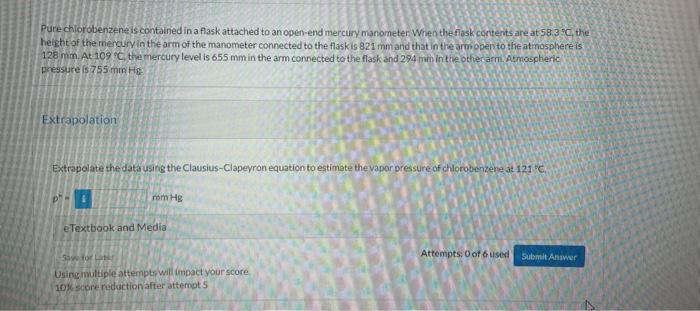
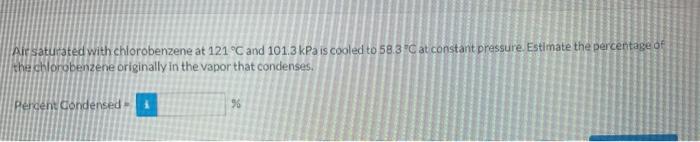
Pure chlorobenzene is contained in a flask attached to an open-end mercury manometer When the flask coritents are at 58.39C, the height of the mercury in the arm of the manometer connected to the flaskis 821mm and that in the arm open to the atmosphere is 128mm. At 1099 C. the mercury level is 655mm in the arm connected to the flask and 294mmm in the other arm. Asmospheric pressure is 755mm He Extrapolate the datausing the Clausius-Clapevron equation to estimate the vapor pressure of chlorobenzene at 121 o C : eTextbook and Media Attempts. 0 of 6 used Using multipie attemptr wil impact your score 10) score raduction after attempt 5 Wir satuitated with chorobenzene at 121C and 101.3kPa is cooled to 58.3C at constant pressure. Estimate the percentage of the chorobenzene originally in the vapor that condenses. Percent Condensed = 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


