Answered step by step
Verified Expert Solution
Question
1 Approved Answer
what was the total mass of CO2 captured by the slab? Consider that the slab acts as a semi-infinite sink for CO2, and that convective
what was the total mass of CO2 captured by the slab? Consider that the slab acts as a semi-infinite sink for CO2, and that convective mass-transfer resistances are eliminated so that cAs cA 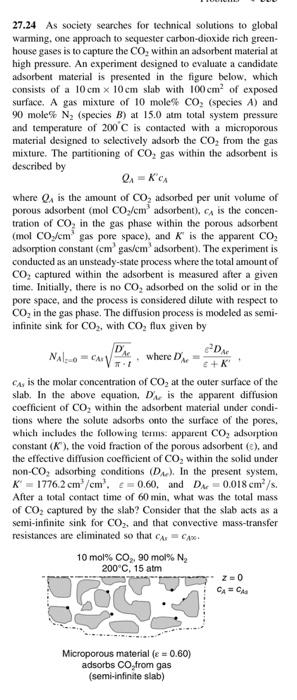
27.24 As society searches for technical solutions to global warming, one approach to sequester carbon dioxide rich green- house gases is to capture the CO, within an adsorbent material at high pressure. An experiment designed to evaluate a candidate adsorbent material is presented in the figure below, which consists of a 10cm x 10cm slab with 100cm? of exposed surface. A gas mixture of 10 mole% CO, (species A) and 90 mole% N, (species B) at 15.0 atm total system pressure and temperature of 200 C is contacted with a microporous material designed to selectively adsorb the CO, from the gas mixture. The partitioning of Co, gas within the adsorbent is described by 0:- KCA where is the amount of Co, adsorbed per unit volume of porous adsorbent (mol CO,/em' adsorbent). A is the concen- tration of Co, in the gas phase within the porous adsorbent (mol CO /cm gas pore space), and is the apparent CO, adsorption constant (cm gas/cm adsorbent). The experiment is conducted as an unsteady-state process where the total amount of CO, captured within the adsorbent is measured after a given time. Initially, there is no CO, adsorbed on the solid or in the pore space, and the process is considered dilute with respect to CO, in the gas phase. The diffusion process is modeled as semi- infinite sink for CO2, with CO, flux given by D D. NALO CAV where D +K Cay is the molar concentration of Co, at the outer surface of the slab. In the above equation, D is the apparent diffusion coefficient of CO, within the adsorbent material under condi- tions where the solute adsorbs onto the surface of the pores. which includes the following terms apparent CO, adsorption constant (K) the void fraction of the porous adsorbent (e), and the effective diffusion coefficient of CO, within the solid under non-Co, adsorbing conditions (D). In the present system, K = 1776.2 cm /cm'. E=0.60, and D =0.018 cm/s. After a total contact time of 60 min, what was the total mass of CO2 captured by the slab? Consider that the slab acts as a semi-infinite sink for CO2, and that convective mass-transfer resistances are eliminated so that C = CAX 10 mol% CO,. 90 mol% N, 200C, 15 atm 20 CACA T. Microporous material (e = 0.60) adsorbs CO from gas (semi-infinite slab) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


