Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What's the answer for these?? 14. If a current of 0.750A is applied to the electrochemical cell above for 30.0min, then the mass of copper
What's the answer for these?? 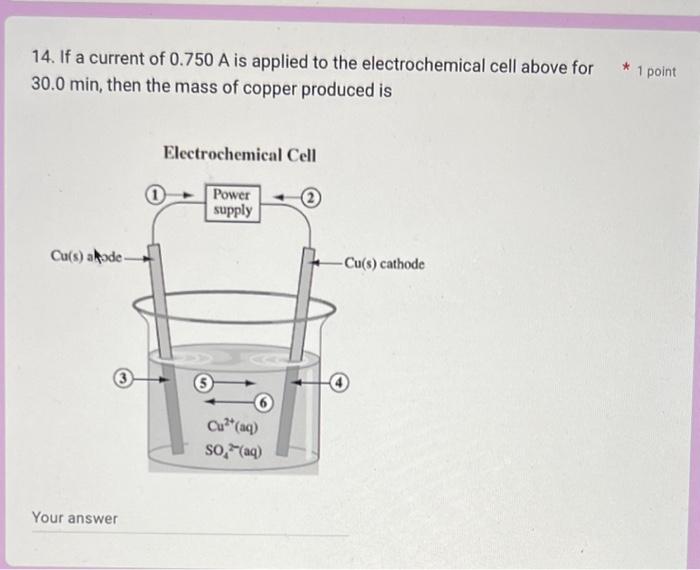
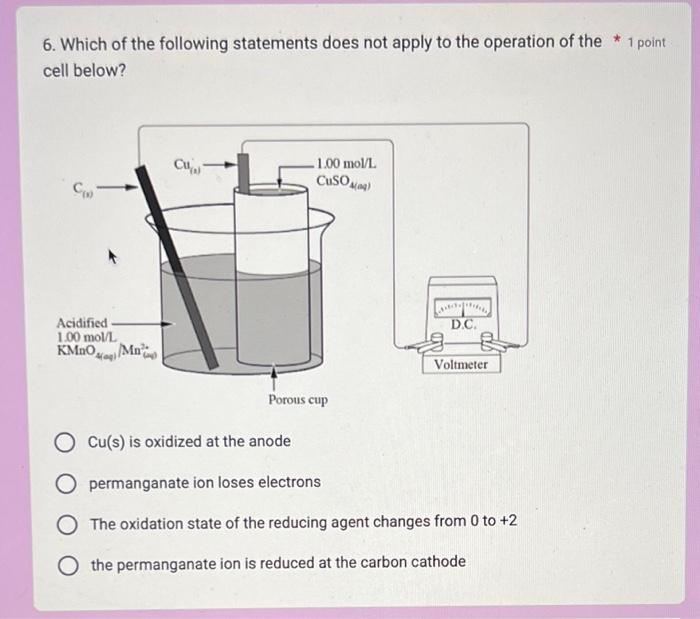

14. If a current of 0.750A is applied to the electrochemical cell above for 30.0min, then the mass of copper produced is Your answer 6. Which of the following statements does not apply to the operation of the * 1 point cell below? Cu(s) is oxidized at the anode permanganate ion loses electrons The oxidation state of the reducing agent changes from 0 to +2 the permanganate ion is reduced at the carbon cathode 11. Use the following balanced equation to answer the next question. The standard electrode potential for the reduction half-reaction is V 2RhCl3(aq)+3Zn(s)3Zn(aq)2++2Rh(s)+12Cl(aq)Enet=+1.20V Your 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


