Question
When a chemical plant needs steam at multiple pressure levels, it is often economical to generate all the steam at a high pressure and then
When a chemical plant needs steam at multiple pressure levels, it is often economical to generate all the steam at a high pressure and then to let the steam down through pressure-reducing turbines to the desired pressure. This principle is illustrated in Figure 8.6. The downside of this approach is that as the exhaust pressure of the turbine increases, the theoretical (and actual)steam requirements increase, meaning that less energy is extracted. To illustrate this point, do the following
a. Estimate the amount of energy extracted from 10,000 kg/h of 58.6 barg steam superheated by 165°C when connected to the following turbines (each 80% efficient):
i. Exhaust pressure is 4″ Hg absolute.
ii. Exhaust pressure is 4.82 barg.
b. Estimate the amount of energy extracted from 10,000 kg/h of saturated, 10.0 barg steam when connected to a turbine (80% efficient) exhausting at 4.82 barg. c. Identify the locations of each of the three turbines described above on Figure 8.6
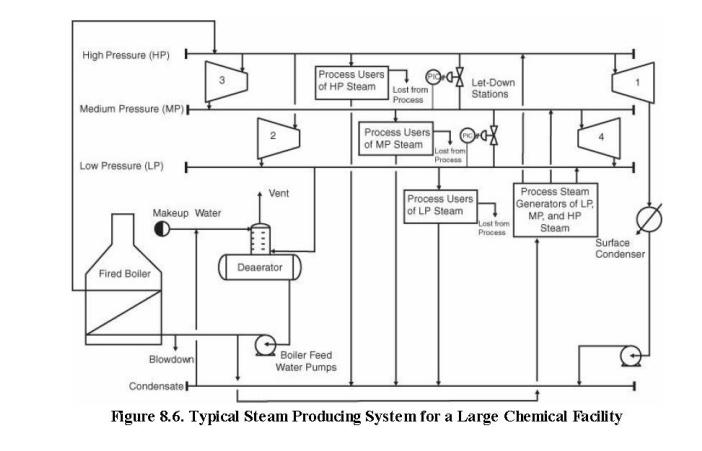
High Pressure (HP) H Medium Pressure (MP) H Low Pressure (LP) H Makeup Water Fired Boiler Blowdown Condensate 2 Vent Deaerator Process Users of HP Steam Boiler Feed Water Pumps Lost from Process Process Users of MP Steam * Lost from Process Let-Down Stations Process Users of LP Steam Lost from Process Process Steam Generators of LP MP, and HP Steam Surface Condenser Figure 8.6. Typical Steam Producing System for a Large Chemical Facility
Step by Step Solution
3.50 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


