Answered step by step
Verified Expert Solution
Question
1 Approved Answer
When a gas follows path 123 on the PV diagram in the figure below, 425 J of energy flows into the system by heat
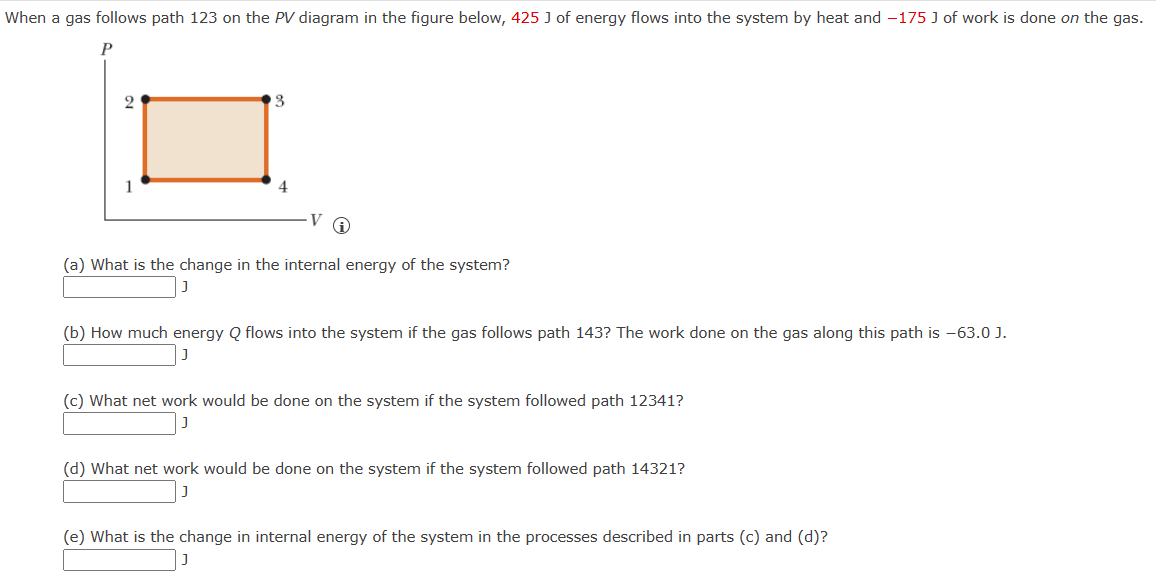
When a gas follows path 123 on the PV diagram in the figure below, 425 J of energy flows into the system by heat and -175 J of work is done on the gas. 3 4 V (a) What is the change in the internal energy of the system? (b) How much energy Q flows into the system if the gas follows path 143? The work done on the gas along this path is -63.0 J. (c) What net work would be done on the system if the system followed path 12341? (d) What net work would be done on the system if the system followed path 14321? (e) What is the change in internal energy of the system in the processes described in parts (c) and (d)?
Step by Step Solution
★★★★★
3.38 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
This problem is related to the first law of thermodynamics which is given by Delta U Q W where Delta U is the change in internal energy of the system ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


