Answered step by step
Verified Expert Solution
Question
1 Approved Answer
When a gas is expanded at constant pressure, the work that is done can be calculated very easily, by W=-PAV. However, this would require
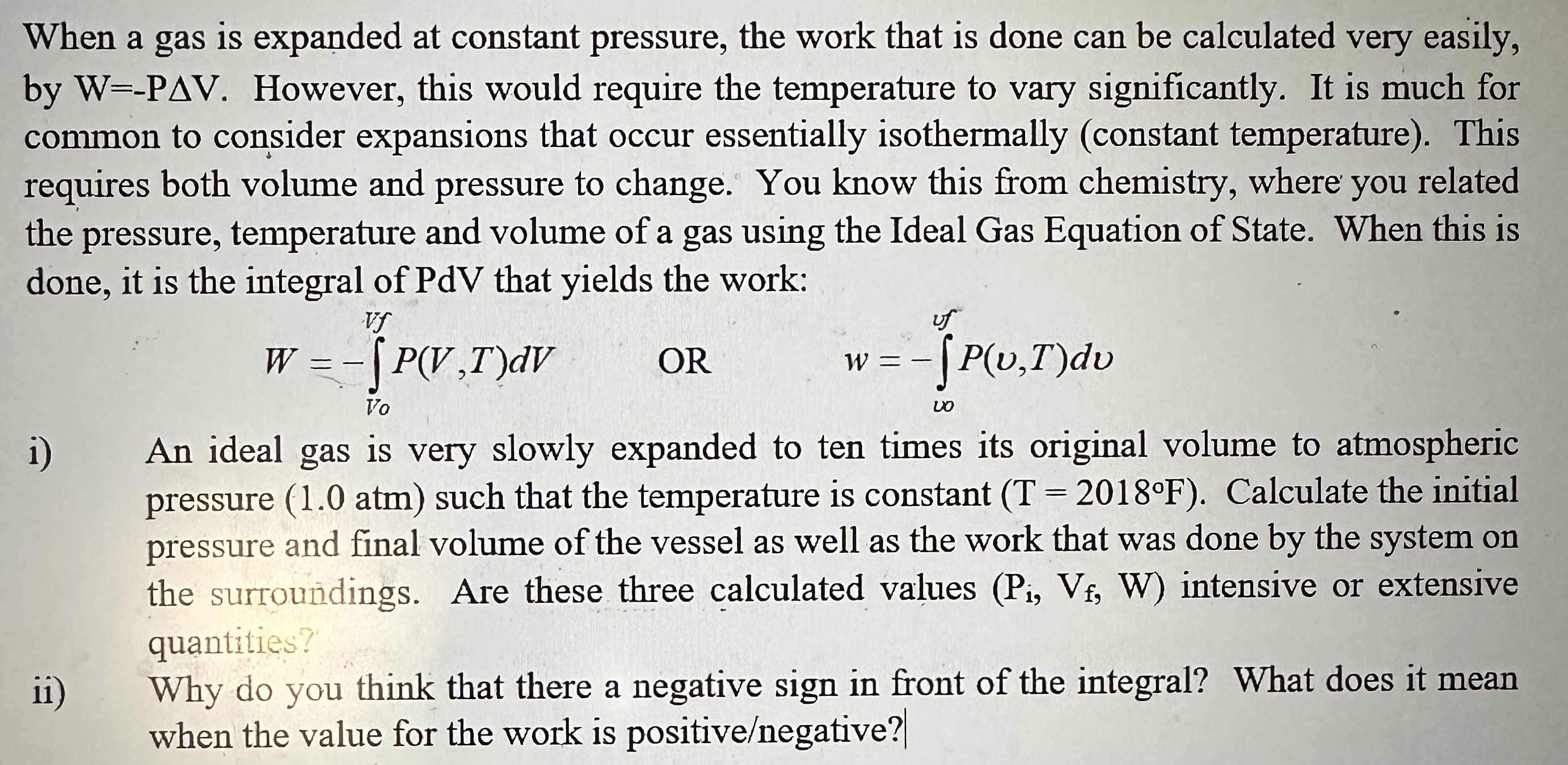
When a gas is expanded at constant pressure, the work that is done can be calculated very easily, by W=-PAV. However, this would require the temperature to vary significantly. It is much for common to consider expansions that occur essentially isothermally (constant temperature). This requires both volume and pressure to change. You know this from chemistry, where you related the pressure, temperature and volume of a gas using the Ideal Gas Equation of State. When this is done, it is the integral of PdV that yields the work: Vf W = = P(V,T)dv Vo OR uf w = - [P(v,T)dv - 00 i) An ideal gas An ideal gas is very slowly expanded to ten times its original volume to atmospheric pressure (1.0 atm) such that the temperature is constant (T = 2018F). Calculate the initial pressure and final volume of the vessel as well as the work that was done by the system on the surroundings. Are these three calculated values (Pi, Vf, W) intensive or extensive quantities? ii) Why do you think that there a negative sign in front of the integral? What does it mean when the value for the work is positive/negative?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


