Question
When a soap bubble is left in a room, air molecules diffuse out from the bubble. If at an instant, the concentrations of air
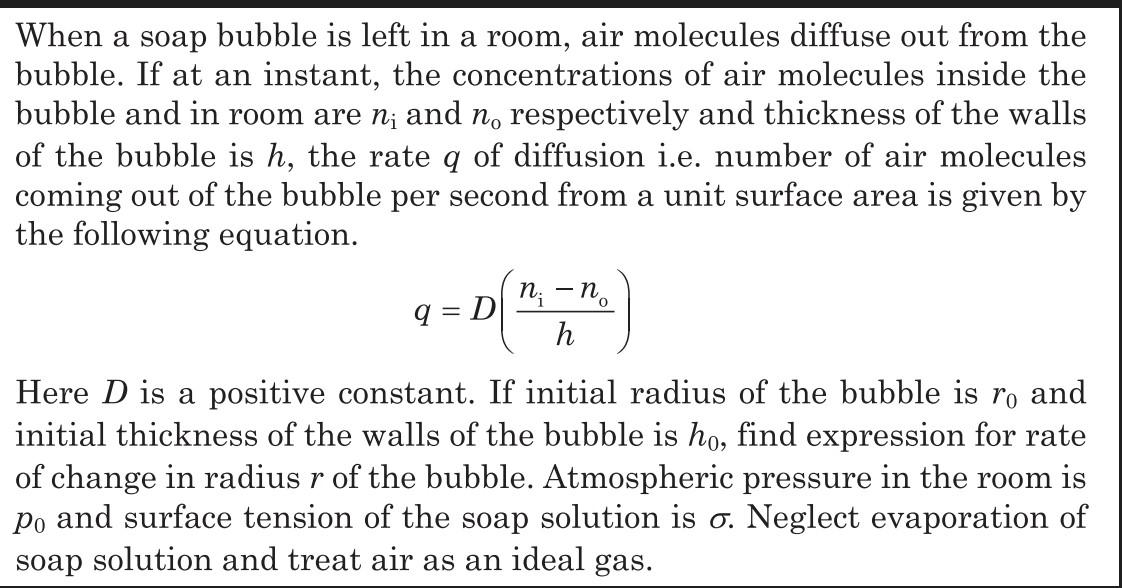
When a soap bubble is left in a room, air molecules diffuse out from the bubble. If at an instant, the concentrations of air molecules inside the bubble and in room are n; and no respectively and thickness of the walls of the bubble is h, the rate q of diffusion i.e. number of air molecules coming out of the bubble per second from a unit surface area is given by the following equation. 9=D ni - n h 0 Here D is a positive constant. If initial radius of the bubble is ro and initial thickness of the walls of the bubble is ho, find expression for rate of change in radius r of the bubble. Atmospheric pressure in the room is Po and surface tension of the soap solution is o. Neglect evaporation of soap solution and treat air as an ideal gas.
Step by Step Solution
3.49 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics Principles with Applications
Authors: Douglas C. Giancoli
7th edition
978-0321869111, 321625927, 9780321733627, 321869117, 9780321625922, 321733622, 978-0321762429
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



