Answered step by step
Verified Expert Solution
Question
1 Approved Answer
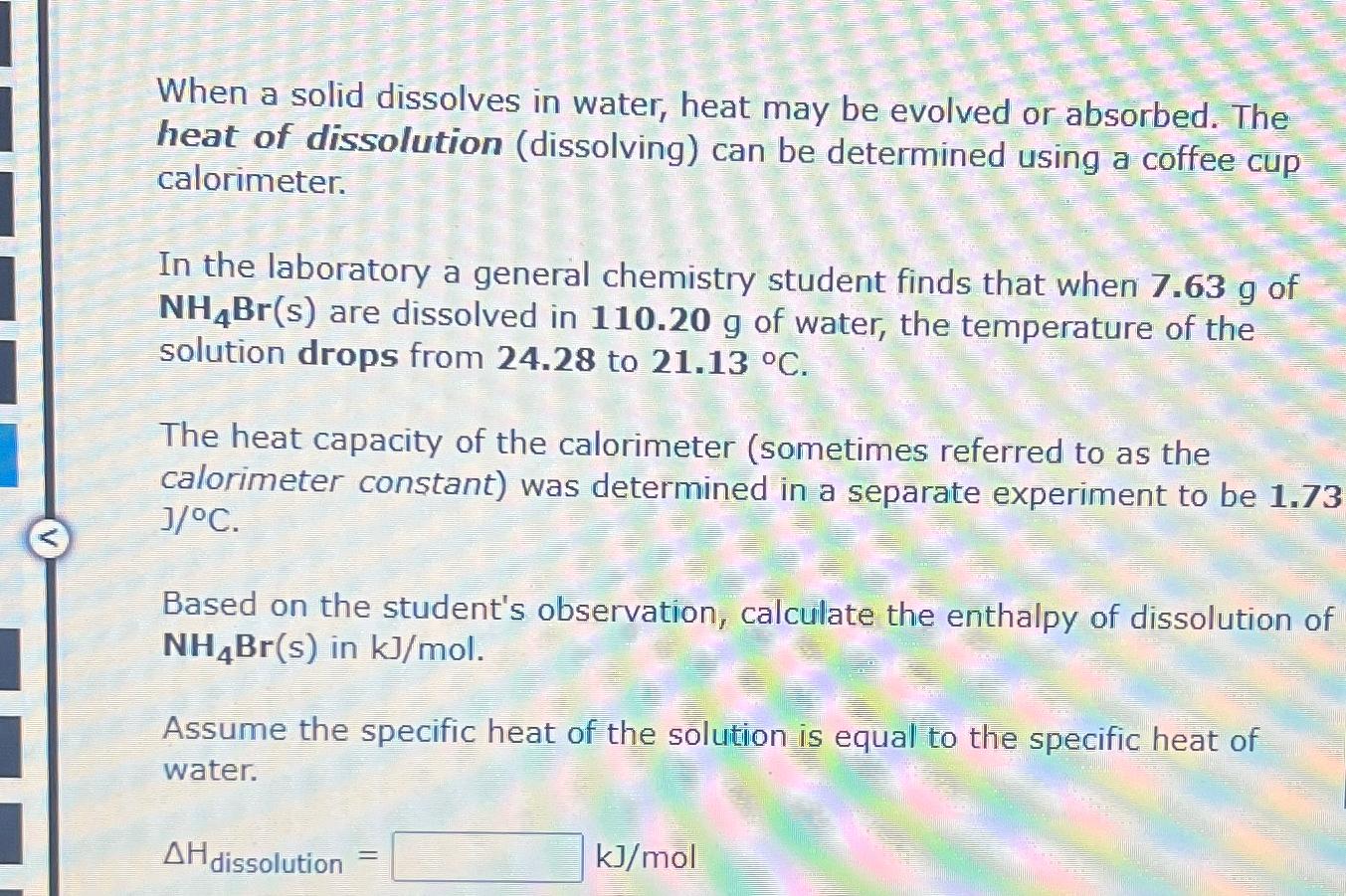
When a solid dissolves in water, heat may be evolved or absorbed . The heat of dissolution ( dissolving ) can be determined using a
When a solid dissolves in water, heat may be evolved or absorbed The heat of dissolution dissolving can be determined using a coffee cup calorimeter.
In the laboratory a general chemistry student finds that when of are dissolved in of water, the temperature of the solution drops from to
The heat capacity of the calorimeter sometimes referred to as the calorimeter constant was determined in a separate experiment to be
Based on the student's observation, calculate the enthalpy of dissolution of in
Assume the specific heat of the solution is equal to the specific heat of water.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


