Question: When sound waves propagate through a gas, the pressure changes are quick enough that the process may be considered adiabatic. As with other compression
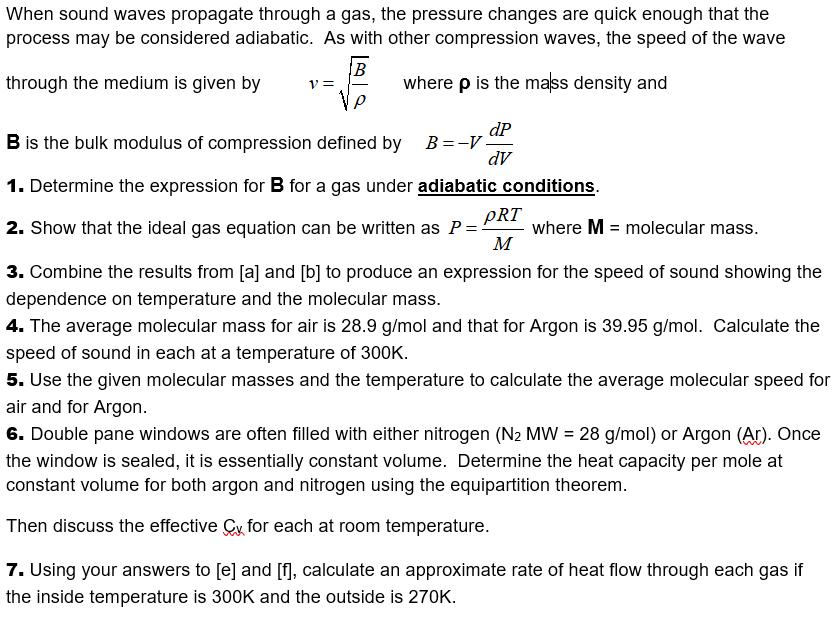
When sound waves propagate through a gas, the pressure changes are quick enough that the process may be considered adiabatic. As with other compression waves, the speed of the wave B V = where p is the mass density and through the medium is given by dP B =-V dV B is the bulk modulus of compression defined by 1. Determine the expression for B for a gas under adiabatic conditions. pRT 2. Show that the ideal gas equation can be written as P= where M = molecular mass. M 3. Combine the results from [a] and [b] to produce an expression for the speed of sound showing the dependence on temperature and the molecular mass. 4. The average molecular mass for air is 28.9 g/mol and that for Argon is 39.95 g/mol. Calculate the speed of sound in each at a temperature of 300K. 5. Use the given molecular masses and the temperature to calculate the average molecular speed for air and for Argon. 6. Double pane windows are often filled with either nitrogen (N2 MW = 28 g/mol) or Argon (Ar). Once the window is sealed, it is essentially constant volume. Determine the heat capacity per mole at constant volume for both argon and nitrogen using the equipartition theorem. Then discuss the effective Cy for each at room temperature. 7. Using your answers to [e] and [], calculate an approximate rate of heat flow through each gas if the inside temperature is 300K and the outside is 270K.
Step by Step Solution
3.37 Rating (156 Votes )
There are 3 Steps involved in it
In thermodynamics an adiabatic pro... View full answer

Get step-by-step solutions from verified subject matter experts


