Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Which graph accurately represents Avogadro's Law? The y-axis is V/n (ratio of volume-to-moles) and the x-axis is n (moles) for a gas sample. The
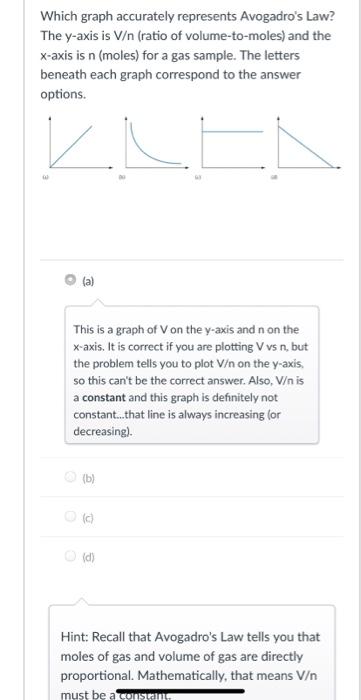
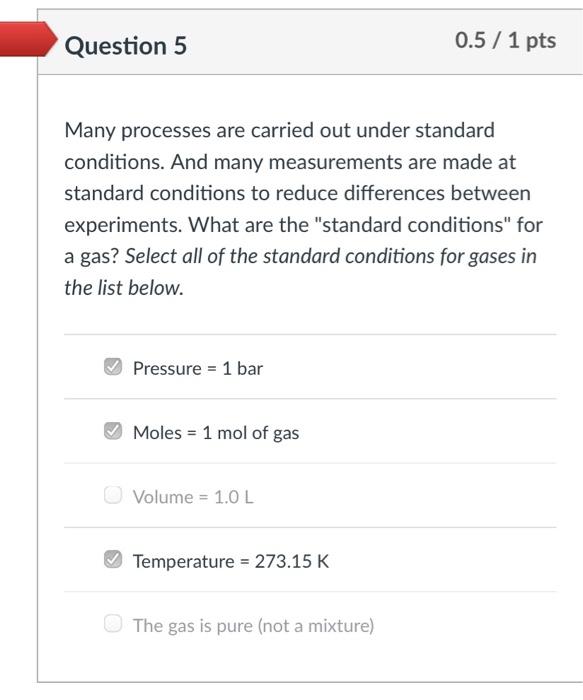
Which graph accurately represents Avogadro's Law? The y-axis is V/n (ratio of volume-to-moles) and the x-axis is n (moles) for a gas sample. The letters beneath each graph correspond to the answer options. KLEN This is a graph of V on the y-axis and n on the x-axis. It is correct if you are plotting V vs n, but the problem tells you to plot V/n on the y-axis, so this can't be the correct answer. Also, V/n is a constant and this graph is definitely not constant.that line is always increasing (or decreasing). O (b) O (c) O (d) Hint: Recall that Avogadro's Law tells you that moles of gas and volume of gas are directly proportional. Mathematically, that means V/n must be aconstant Question 5 0.5 /1 pts Many processes are carried out under standard conditions. And many measurements are made at standard conditions to reduce differences between experiments. What are the "standard conditions" for a gas? Select all of the standard conditions for gases in the list below. Pressure = 1 bar Moles = 1 mol of gas Volume = 1.0 L Temperature = 273.15 K The gas is pure (not a mixture)
Step by Step Solution
★★★★★
3.52 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
Question 4 Answer c According to Avogadros law V n V volume n moles Vn constant Volume by m...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


