Question
Which of the following aqueous solutions has the highest osmotic pressure at 25C (assume 100% dissociation for all soluble ionic compounds)? A) 0.15M K3PO4
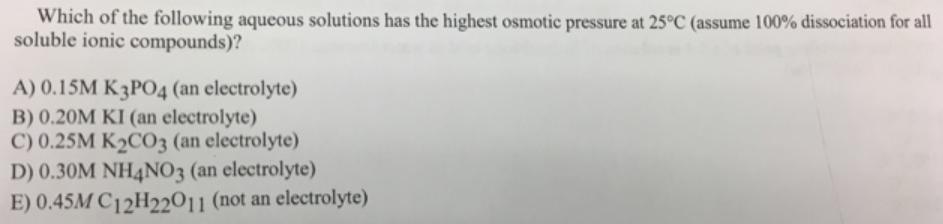
Which of the following aqueous solutions has the highest osmotic pressure at 25C (assume 100% dissociation for all soluble ionic compounds)? A) 0.15M K3PO4 (an electrolyte) B) 0.20M KI (an electrolyte) C) 0.25M K2CO3 (an electrolyte) D) 0.30M NH4NO3 (an electrolyte) E) 0.45M C12H22011 (not an electrolyte)
Step by Step Solution
3.18 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
TT icsT T asmotic pressure iz vorit hoff factor Cmolasity S 00821 atm mo T Tem...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



