Question
Which of the following diatomic species are paramagnetic and which are diamagnetic? A blank molecular orbital diagram (Figure 2) has been provided to help
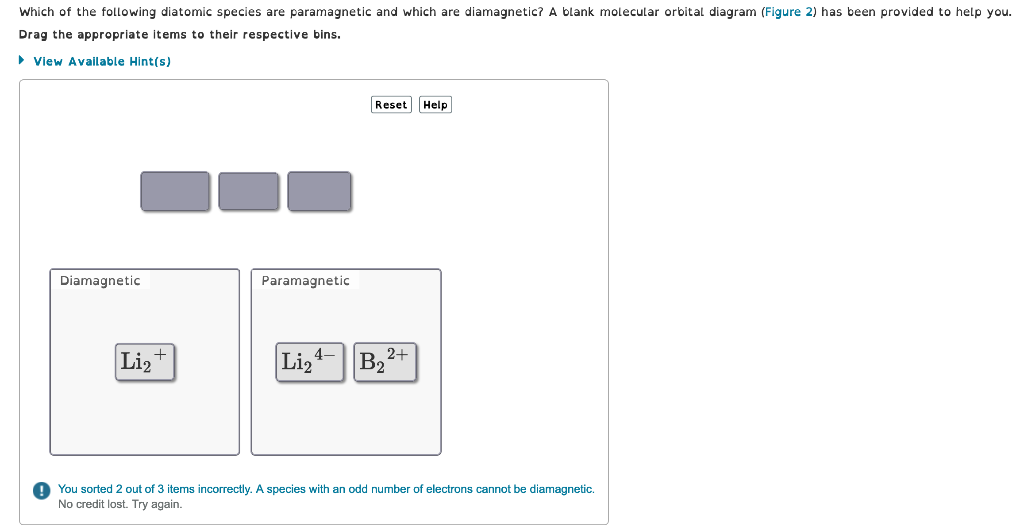
Which of the following diatomic species are paramagnetic and which are diamagnetic? A blank molecular orbital diagram (Figure 2) has been provided to help you. Drag the appropriate items to their respective bins. View Available Hint(s) Diamagnetic Liz Paramagnetic Li Reset 2+ B Help You sorted 2 out of 3 items incorrectly. A species with an odd number of electrons cannot be diamagnetic. No credit lost. Try again.
Step by Step Solution
3.36 Rating (168 Votes )
There are 3 Steps involved in it
Step: 1
Li 2 and B 2 2 are paramagnetic while Li 2 4 is diamagnetic In chemistry param...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



