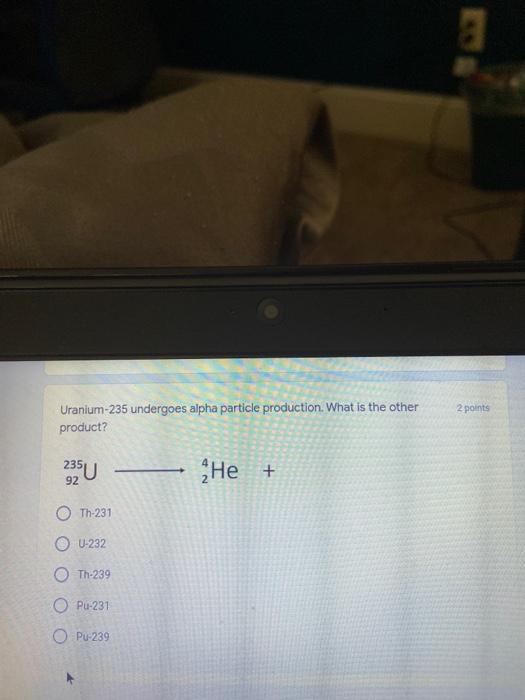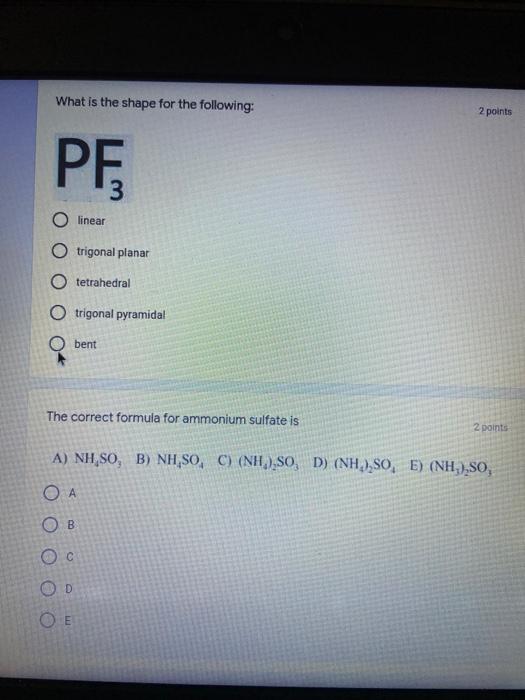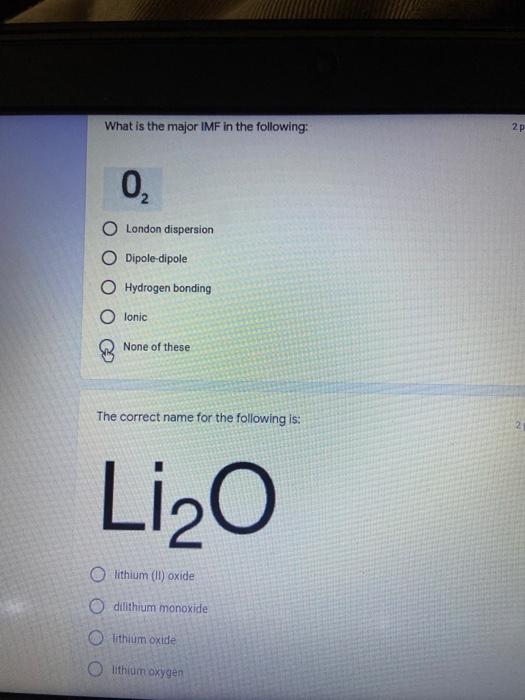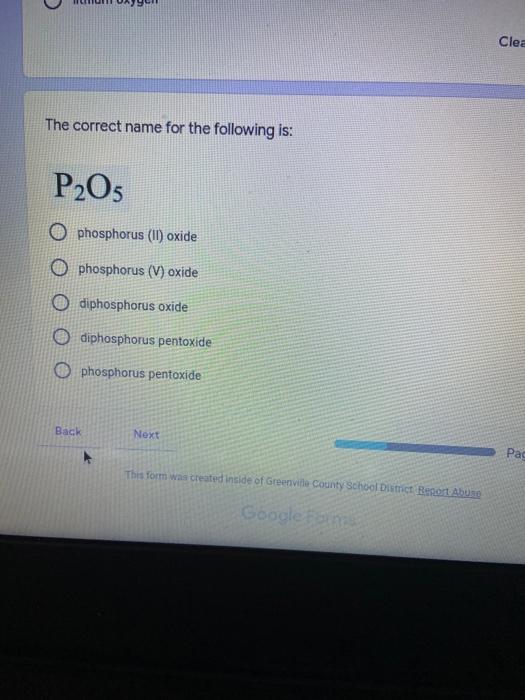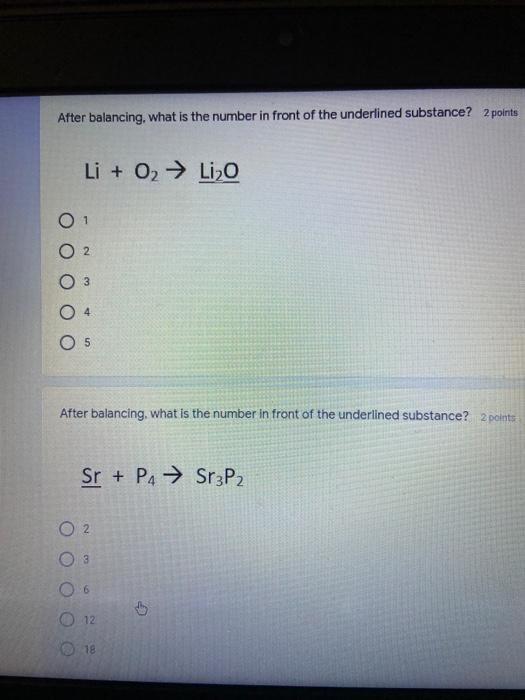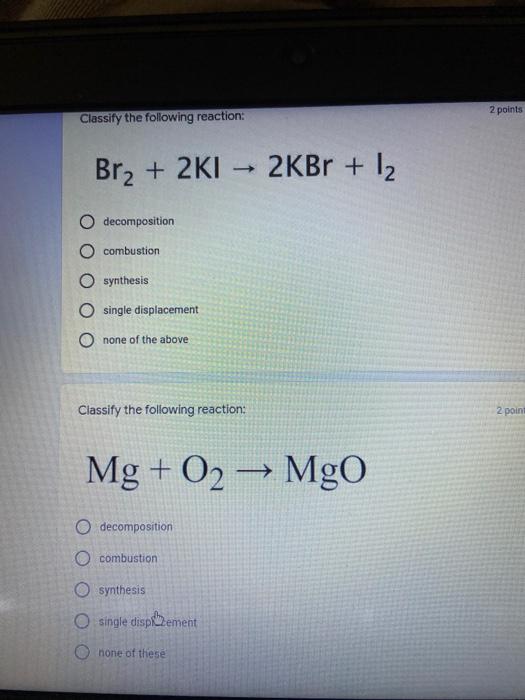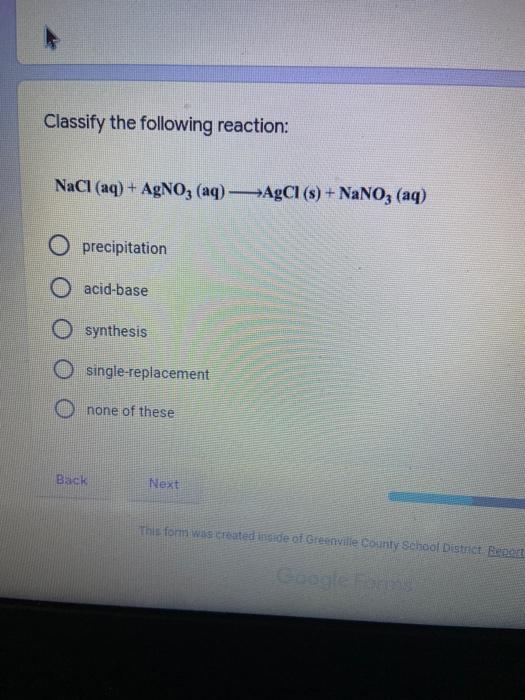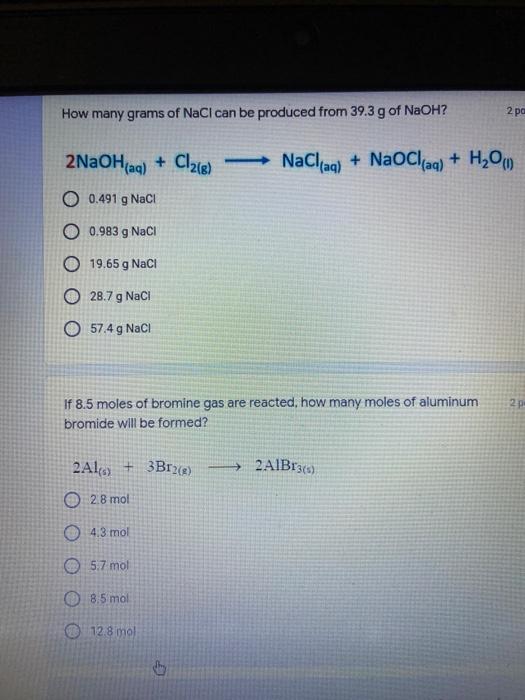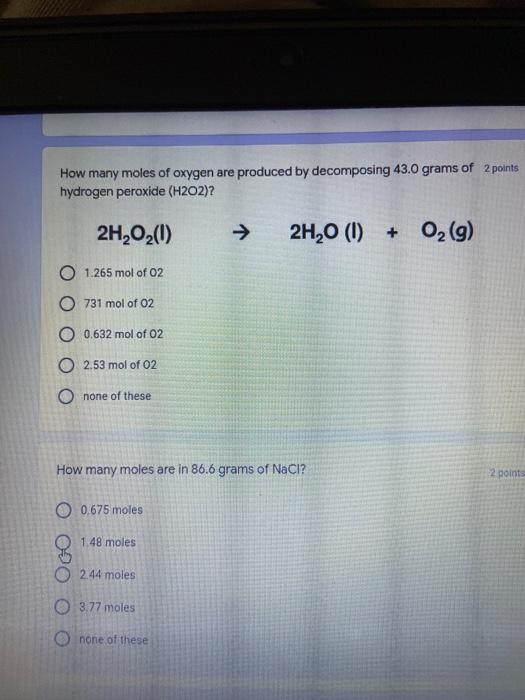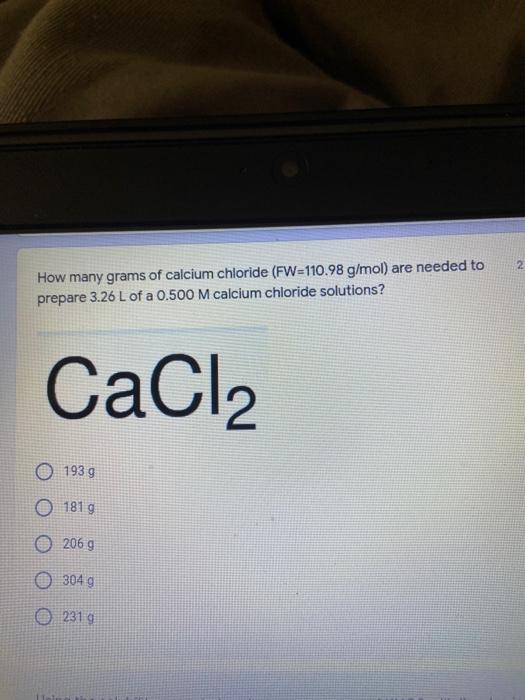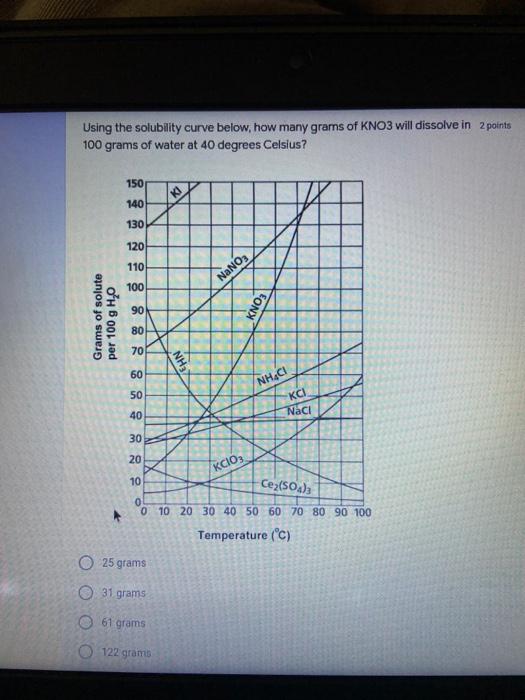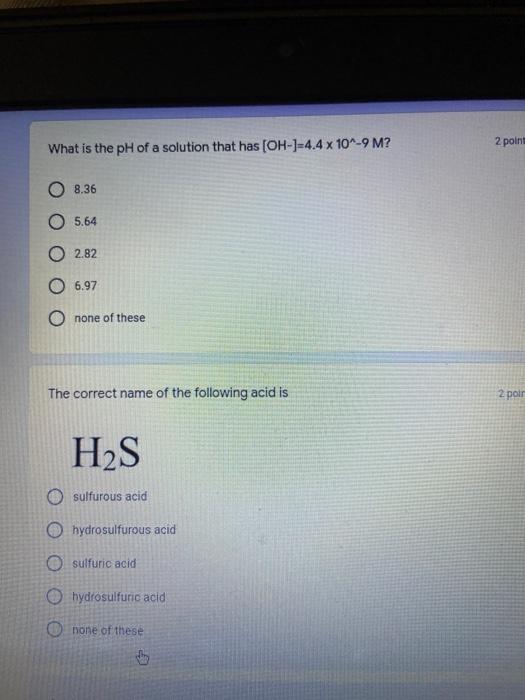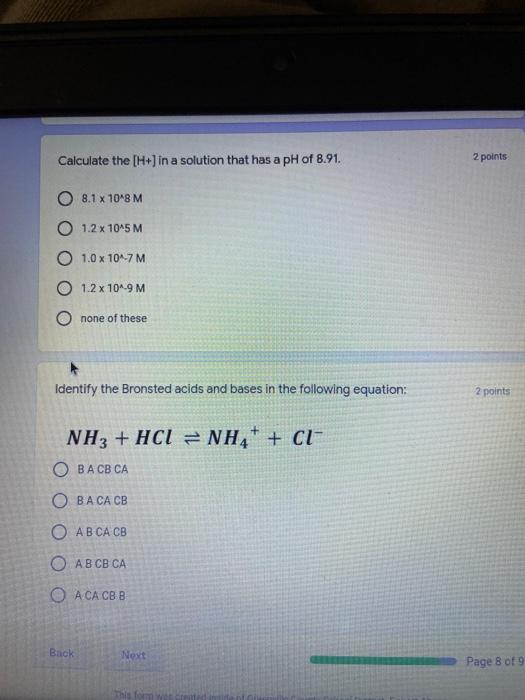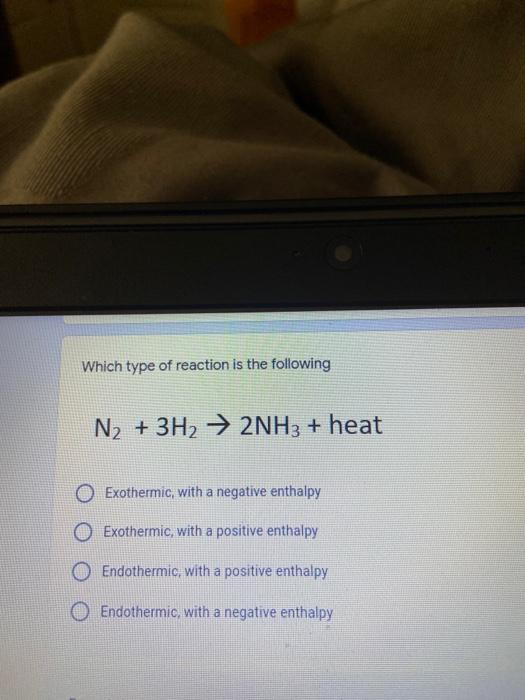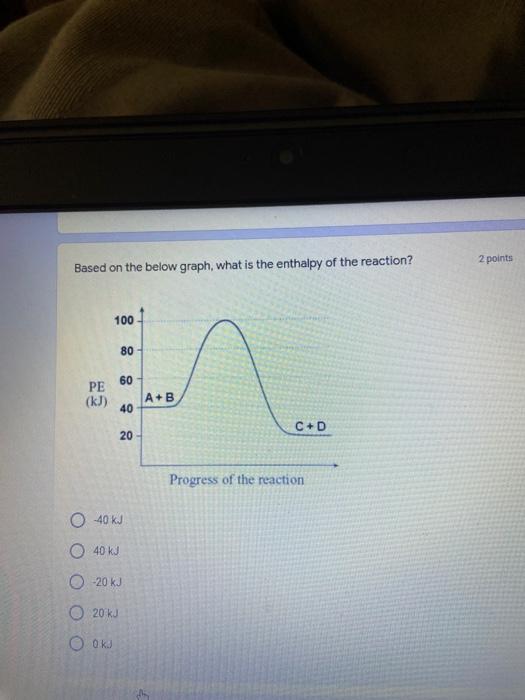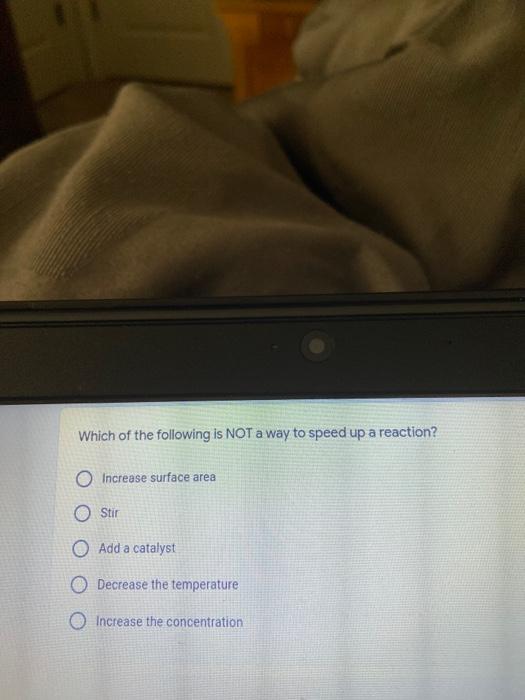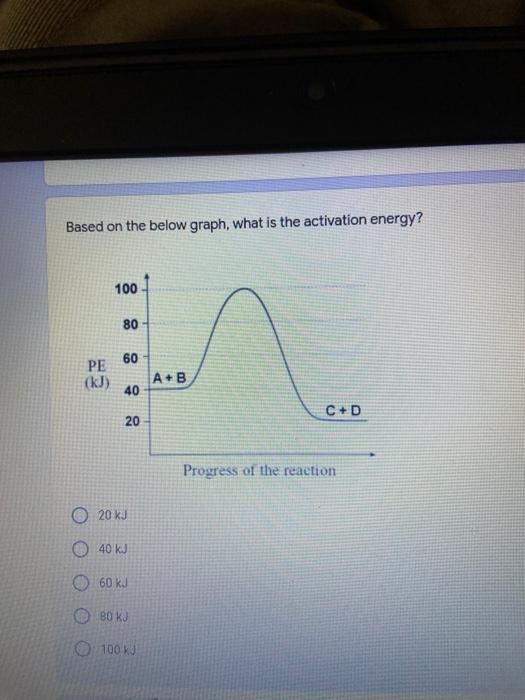
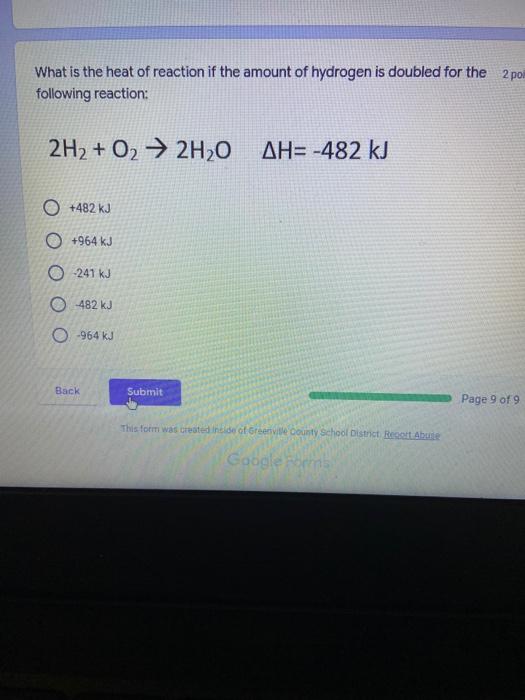
Which of the following elements is an alkali metal? 2 points Ca Cu Fe Na SC Which of the following electron configurations are written INCORRECTLY? 2 points 1. Ca: 1s22s22p383p45 II. Mg: 1s 2s 2p 3s III. V: 1s 2s22p%3s 3p 4s 3d IV. As: 1s 2s 2p"3s 3p4s 3d4p V.P: 1s 2s 2p 3p II IV V Clear selection Which of the following is a noble gas? 2 points O Argon Nitrogen Hydrogen O Oxygen O Carbon dioxide 2 points Which of the following is ranked in the order of LARGEST to SMALLEST atomic radius? Rb>Mn>S>Ge>F OF>$>Ge>Mn>Rb MnRb>F>S>Ge Rb>Ge>Mn>>S Rb>Mn>Ge-S>F a 2 points Uranium-235 undergoes alpha particle production. What is the other product? 2350 He + e 92 OTH-231 U-232 OTH-239 Pu-231 O Pu-239 How many protons, electrons, and neutrons, respectively, does Al-27 have 2 points as an ION? AB+ 13,13,14 13,10,14 13,13,27 13,10,27 13,13,13 Which of the following is a nonmetal? 2 points Chromium Cesium O Carbon Calcium O Copper 2 points Which of the following elements have the LOWEST electronegativity? Phosphorus Sulfur Arsenic Selenium The half-life of a radioactive nuclide is 2 points that period of time in which 25% of the original number of atoms undergoes O radioactive decay O the time at which the isotope becomes nonradioactive. O that period of time in which 50% of the original number of atoms undergoes radioactive decay O the period of time it takes to reduce the radioactivity by 100% none of the above Which statement best describes the process of nuclear fission? 2 points The splitting of a large nuclei to release a large amount of energy and two smaller nuclei. The splitting of a small nuclei to release a small amount of energy and two smaller nuclei. The combination of two large nuclei to release a large amount of energy and one larger nuclei. The combination of two small nuclei to release a large amount of energy and one larger nuclei. The combination of two small nuclei to absorb a large amount of energy and one larger nuclei. The number of neutrons in one atom of Hg-202 is 2 points 80 O 202 O 122 0282 none of these What is the major IMF in the following: K20 London dispersion O Dipole-dipole Hydrogen bonding lonic None of these What is the shape for the following: CH4 O linear trigonal planar tetrahedral trigonal pyramidal bent Which of the following has a double bond? (Draw the Lewis Structure in order to determine this.) 2 points A) H0 B) NH, C) 0, D) CO E) H,S B OD The correct formula for titanium (IV) oxide is 2 points D) Ti,O E) 1,0 A) Tio. B) Ti,o C) TO, OB T7 What is the shape for the following: 2 points P 3 O linear O trigonal planar tetrahedral O trigonal pyramidal bent The correct formula for ammonium sulfate is 2 points A) NHSO, B) NH SO, C) (NH),SO, D) (NH),SO, E) (NH,),SO, O A OB OD What is the major IMF in the following: 2p 0, London dispersion O Dipole dipole Hydrogen bonding lonic None of these The correct name for the following is: Li2O Olithium (II) oxide dilithium monoxide lithium oxide O lithium oxygen Clea The correct name for the following is: P2O5 O phosphorus (11) oxide O phosphorus (V) oxide O diphosphorus oxide O diphosphorus pentoxide phosphorus pentoxide Back Next This form was created inside of Greenville County School District Besort Abuso Google Fans After balancing, what is the number in front of the underlined substance? 2 points Li + O2 Li2O 0 1 2 3 4 After balancing, what is the number in front of the underlined substance? 2 points Sr + PA Sr3P2 O 2 O 3 6 O 12 2 points Classify the following reaction: Br2 + 2K1 2KBr + 12 decomposition combustion synthesis single displacement O none of the above Classify the following reaction: 2 point Mg + O2 MgO O decomposition O combustion synthesis O single disprement none of these Classify the following reaction: NaCl (aq) + AgNO3 (aq) AgCl (s) + NaNO3 (aq) precipitation O acid-base O synthesis single-replacement none of these Back Next This form was created inside of Greenville County School District Report How many grams of NaCl can be produced from 39.3 g of NaOH? 2 po 2NaOH(aq) + Cl2(8) - NaCl(aq) + NaOCl(aq) + H201) O 0.491 g Naci O 0.983 g NaCl O 19.65 g Naci 28.7 g Naci O 57.4 g NaCl 2p If 8.5 moles of bromine gas are reacted, how many moles of aluminum bromide will be formed? 2A1B13(0) 2Al) + 3B12(e) 2.8 mol 4.3 mol 57 mol O 8.5 mol 12.8 mol How many moles of oxygen are produced by decomposing 43.0 grams of 2 points hydrogen peroxide (H2O2)? 2H,02(0) 2H20 (1) + O2(g) 1.265 mol of 02 O 731 mol of 02 O 0.632 mol of 02 O 2.53 mol of 02 O none of these How many moles are in 86.6 grams of Naci? 2 points O 0.675 moles 1.48 moles 0 2.44 moles O 3.77 moles O none of these How many significant figures are in the number 0.00204? O 2 3 O 5 06 How many atoms are in 90.7 g of nickel? O 1.55 O 5.32 x 1043 O 9.30 x 10^23 05.46 x 10-25 O none of these Back Next Pag The formats of Green County School District Resort AS 2 points Consider a gas at 1.00 atm in a 5.00 L container at 20 degrees Celsius. What pressure does the gas exert when transferred to a volume of 3.56 L at 43 degrees Celsius? (Remember to convert temperature to Kelvin.) 03.02 atm O 1.30 atm O 0.371 atm O 1.51 atm O 0.768 atm Convert the following for pressure: 1.224 atm- torr 2 points O 930,2 O 620,9 O 1.611x1043 O 17.99 1.224 Clear selection Celsius Convert the following for temperature: 311 K = 2 points 38 Celsius 584 Celsius O 1.14 Celsius 273 Celsius O none of these If you have a 2.0 Liter balloon at room temperature and then put it in the freezer, what will happen to the volume? 2 points It increases It decreases O' It remains the same Back Next Page 6 of 9 This form was created in of Greene County School Olunteer 2 points What is the minimum volume of a 2.06 M NaOH solution needed to make 150.0 mL of a 0.800 M NaOH solution? 375 ml 29.1 ml O 70.3 mL O 120.mL O 58.3 mL Which of the following is NOT a solid when put into water? 2 points A) AgCl B) CaCO, C) Caso D) AICI: OB Oc OD How many grams of calcium chloride (FW=110.98 g/mol) are needed to prepare 3.26 L of a 0.500 M calcium chloride solutions? CaCl2 O 1939 1819 2069 304 g 02319 Using the solubility curve below, how many grams of KNO3 will dissolve in 2 points 100 grams of water at 40 degrees Celsius? 150 4 140 130 120 110 100 NaNO3 KNO 90 Grams of solute per 100 g H2O 80 70 NH 60 NHC 50 KCI 40 Naci 30 20 KCIO3 10 Cez(SO4)3 0 0 10 20 30 40 50 60 70 80 90 100 Temperature (C) O 25 grams O 31 grams O 61 grams 122 grams 2 point What is the pH of a solution that has (OH-]=4.4 x 10^-9 M? 8.36 5,64 2.82 O 6.97 O none of these The correct name of the following acid is 2 polr H2S O sulfurous acid hydrosulfurous acid O sulfuric acid hydrosulfuric acid Onone of these Calculate the [H+) in a solution that has a pH of 8.91. 2 points o8.1 x 108 1.2 x 105 1.0 x 1047 1.2 10-9 none of these Identify the Bronsted acids and bases in the following equation: 2 points NH3 + HCl = NH4* + Cl - Back Next Page 8 of 9 Which type of reaction is the following N2 + 3H2 2NH3 + heat Exothermic, with a negative enthalpy Exothermic, with a positive enthalpy Endothermic, with a positive enthalpy Endothermic, with a negative enthalpy 2 points Based on the below graph, what is the enthalpy of the reaction? 100 80 60 PE (kJ) A+B 40 C+D 20 Progress of the reaction O 40 kJ 40 kJ O 20kJ O 20k Ook Which of the following is NOT a way to speed up a reaction? Increase surface area O Stir Add a catalyst O Decrease the temperature Increase the concentration Based on the below graph, what is the activation energy? 100 80 60 A PE (kJ) A+B 40 C+D 20 Progress of the reaction O 20 kJ 040 kJ 60 kJ BOKU 100 KU Solid X is placed in contact with solid Y. Heat will flow spontaneously from 2 points X to Y when A) Xis 20'C and Y is 20c B) X is 10' and Y is sc C) X is -25C and Yis-10'c D) X is 25C and Y is 30'c OA B OD Perform the following conversion: 273 cal = J 2 points 65.20 1140 O 1.143 65200 O 0.0153 What is the What is the heat of reaction if the amount of hydrogen is doubled for the 2 por following reaction: 2H2 + O2 2H2O AH= -482 kJ +482 kJ +964 kJ 241 kJ O 482 kJ 0-964 Back Submit Page 9 of 9 This form was created in de groene Dounty School District Resort Abuse Google