Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Which of the following is diamagnetic? Select one: a. Fe b. Sc C. Zn d. B e. Ti Which one of the following is paramagnetic?






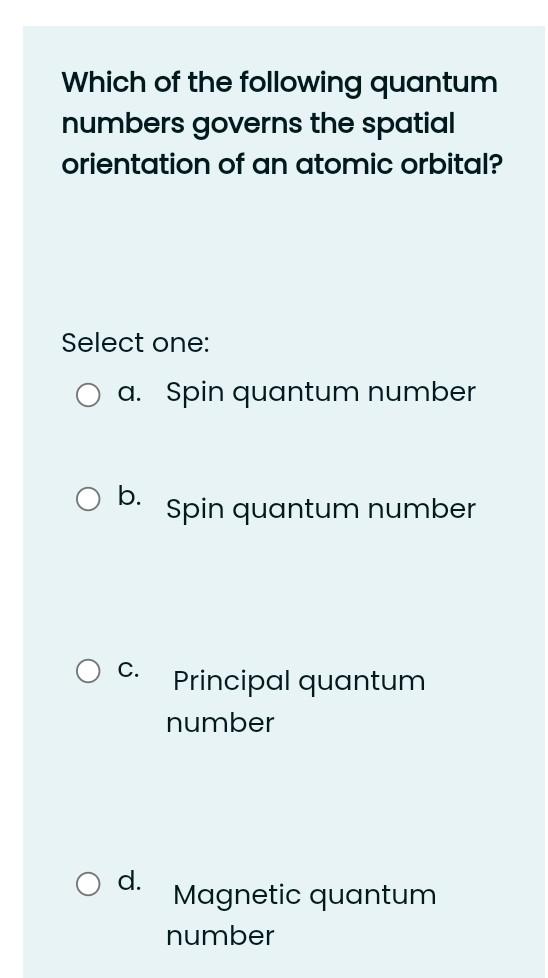

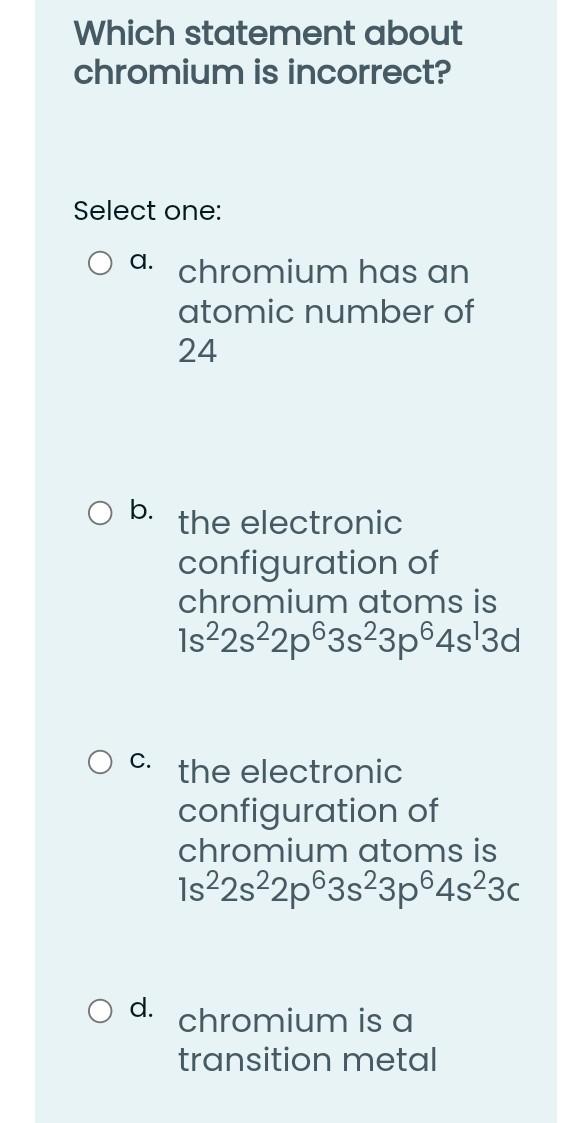

Which of the following is diamagnetic? Select one: a. Fe b. Sc C. Zn d. B e. Ti Which one of the following is paramagnetic? Select one: a. Ne O b. Be C. Fe O d. Zn Which orbitals cannot exist Select one: a. 3d b. 6s c. 4p d. 3f O e. 41 For a principle quantum number, "n", equal to 2, what is the total electron capacity of that level? Select one: g. 16 O b. 8 C. 4. O d. 2 What is the correct representation for an orbital which has an "n" value of 4 and an "L" value of 2? Select one: a. 4d O b. 4p C. 4s d. 4f How many orbitals can have the following quantum numbers, n = 3,1 = 1, m = 0? = Select one: a. 1 b. 2 C. 4 d. 3 Which of the following quantum numbers governs the spatial orientation of an atomic orbital? Select one: a. Spin quantum number b. Spin quantum number C. Principal quantum number Magnetic quantum number How many orbitals are in the 4p sublevel? Select one: a. 2 b. 1 C. 3 d. 4 Which statement about chromium is incorrect? Select one: a. chromium has an atomic number of 24 b. the electronic configuration of chromium atoms is 1s22s22p63s23p645'3d C. the electronic configuration of chromium atoms is 1s22s22p63s23p64s23 chromium is a transition metal Which of the following atoms has the greatest number of unpaired electrons? Select one: : a. Sc b. Mn C. Ti d. V
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


