Question
Which of the following samples has a larger mass? O 19.3 g of Au 8.92 10 atoms of Pt Identify the molecular geometry around
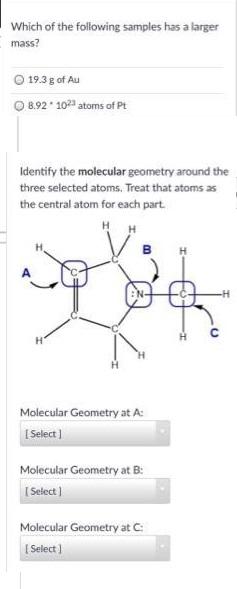
Which of the following samples has a larger mass? O 19.3 g of Au 8.92 10 atoms of Pt Identify the molecular geometry around the three selected atoms. Treat that atoms as the central atom for each part. H H H EN- Molecular Geometry at A: I Select ) Molecular Geometry at B: I Select ) Molecular Geometry at C: I Select )
Step by Step Solution
3.42 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
Ans 9 Mass of Gold 193 g 892 10 atoms of platinum will be equivalent to 892...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Accounting A Practical Approach
Authors: Jeffrey Slater, Brian Zwicker
11th Canadian Edition
132564440, 978-0132564441
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



