Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Which of the following statements about Lewis structures is FALSE? An octet is when an atom has 8 valence electrons. A covalent bond occurs

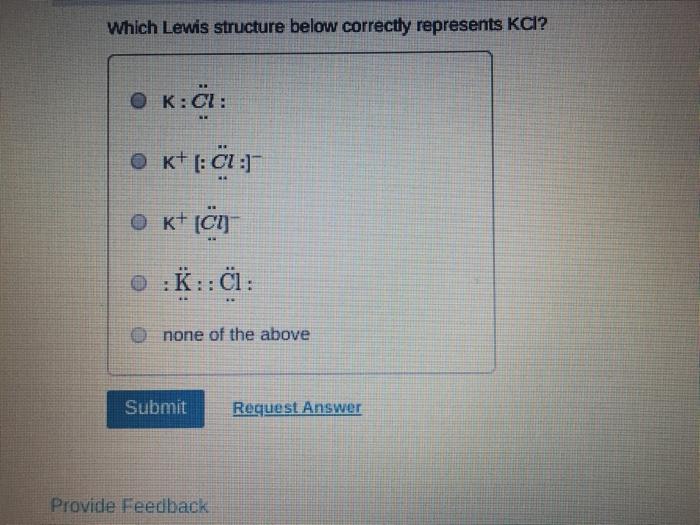
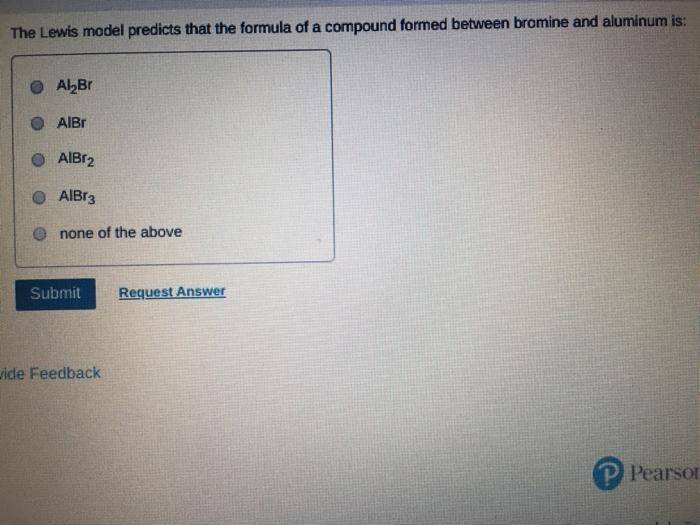
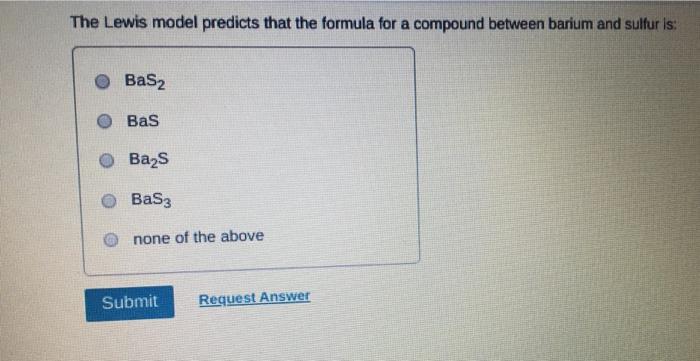
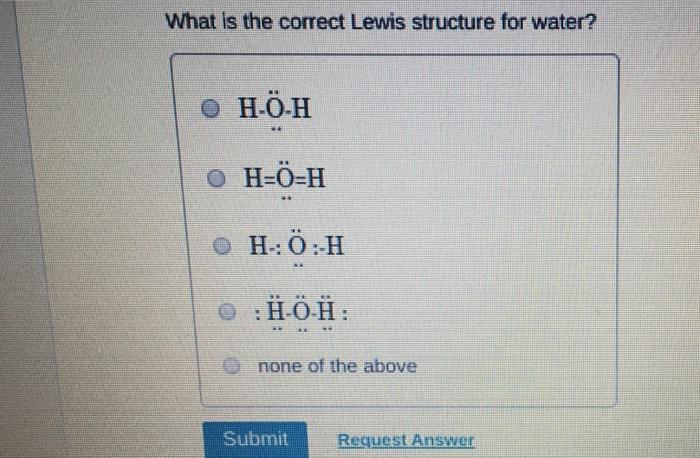
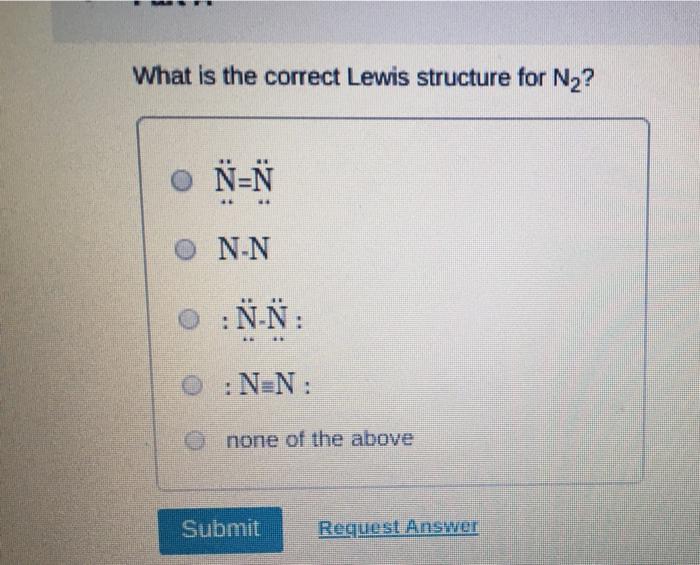
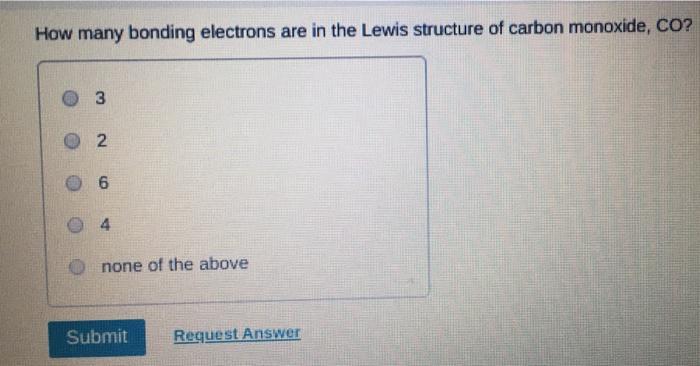

Which of the following statements about Lewis structures is FALSE? An octet is when an atom has 8 valence electrons. A covalent bond occurs when electrons are shared. An ionic bond occurs when electrons are transferred. A duet is a stable electron configuration for helium. All of the above statements are true. Submit Provide Feedback Request Answer Which Lewis structure below correctly represents KCI? K: Cl: K+ [: C:] K+ [CI] :K::C: none of the above Submit Request Answer Provide Feedback The Lewis model predicts that the formula of a compound formed between bromine and aluminum is: AlBr AlBr AlBr 0 AlBr3 none of the above Submit Request Answer ide Feedback P Pearson The Lewis model predicts that the formula for a compound between barium and sulfur is: BaS Bas BaS BaS3 none of the above Submit Request Answer What is the correct Lewis structure for water? OH--H OH--H OH: :-H HOH: none of the above Submit Request Answer What is the correct Lewis structure for N? 0 N=N N-N : - : : N=N: none of the above Submit Request Answer How many bonding electrons are in the Lewis structure of carbon monoxide, CO? 3 2 6 none of the above Submit Request Answer 3- The total number of electrons to be counted for the Lewis structure of the PO4 polyatomic ion is: 26. 29. 8. 32. none of the above Submit Request Answer
Step by Step Solution
★★★★★
3.42 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
1lewis structure is a very simplified representation of the valence shell electrons in a moleculeIt ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


