Question
Which of the following statements is true? O A neutral Mg atom having electron configuration [Ne]4s? cannot emit photons. O The 1s electrons of
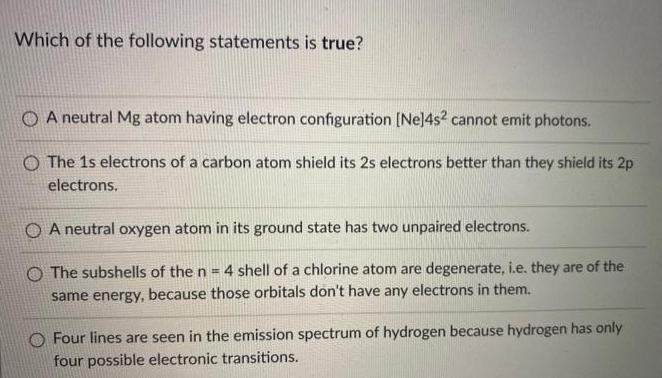
Which of the following statements is true? O A neutral Mg atom having electron configuration [Ne]4s? cannot emit photons. O The 1s electrons of a carbon atom shield its 2s electrons better than they shield its 2p electrons. O A neutral oxygen atom in its ground state has two unpaired electrons. O The subshells of the n = 4 shell of a chlorine atom are degenerate, i.e. they are of the same energy, because those orbitals don't have any electrons in them. O Four lines are seen in the emission spectrum of hydrogen because hydrogen has only four possible electronic transitions.
Step by Step Solution
3.40 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Accounting concepts and applications
Authors: Albrecht Stice, Stice Swain
11th Edition
978-0538750196, 538745487, 538750197, 978-0538745482
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



