Question
Identify the 'best' Lewis structure based on the guidelines presented in this class. The formal charges have been omitted. O: :0-S-O: .O: 0=s-8: :6-s=0
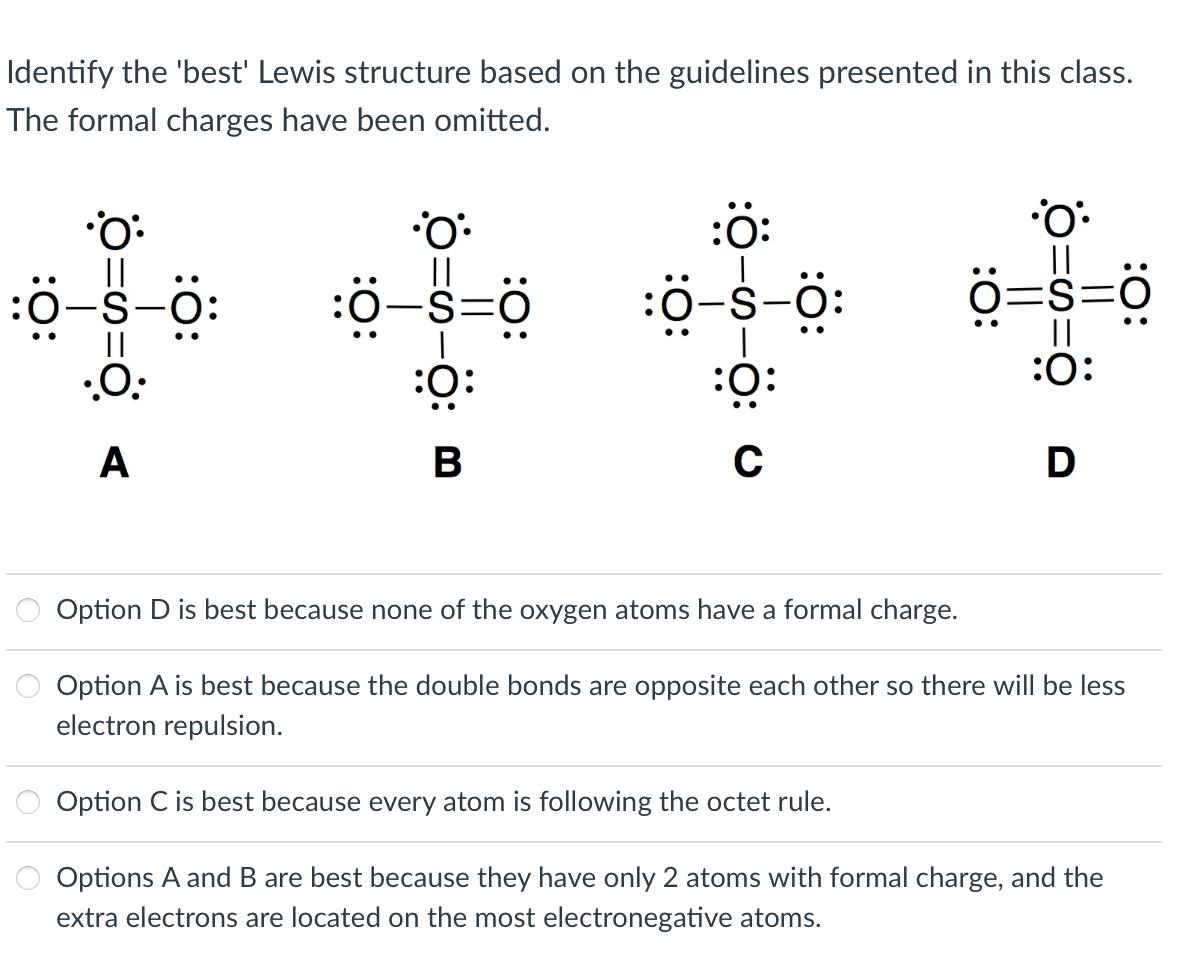
Identify the 'best' Lewis structure based on the guidelines presented in this class. The formal charges have been omitted. O: :0-S-O: .O: 0=s-8: :6-s=0 B :O: :040: :: C Option D is best because none of the oxygen atoms have a formal charge. O: =s=0 || :O: Option C is best because every atom is following the octet rule. D Option A is best because the double bonds are opposite each other so there will be less electron repulsion. Options A and B are best because they have only 2 atoms with formal charge, and the extra electrons are located on the most electronegative atoms.
Step by Step Solution
3.49 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1
The Lewis structure that is the best representation for OCS is Option B Heres why 1 The total numbe...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
13th Edition
0321910419, 978-0321910417
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



