Question
Which of these equations is the balanced reaction between sulfurous acid and magnesium hydroxide? HSO3(0) + MgOH(s) MgSO4(s) + 2 HO() - HS(g) +
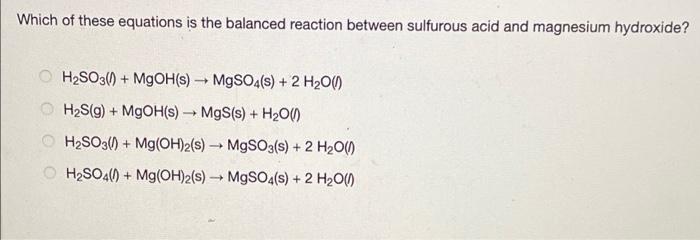
Which of these equations is the balanced reaction between sulfurous acid and magnesium hydroxide? HSO3(0) + MgOH(s) MgSO4(s) + 2 HO() - HS(g) + MgOH(s) MgS(s) + HO(1) HSO3(0) + Mg(OH)2(s) MgSO3(s) + 2 HO() HSO4()+ Mg(OH)2(s) MgSO4(s) + 2 HO()
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
Answer HSO MgOH MgSO 2HO 0 s Explanation Sulfuro...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Applied Statistics For Public And Nonprofit Administration
Authors: Kenneth J. Meier, Jeffrey L. Brudney, John Bohte
9th Edition
1285737237, 978-1285974521, 1285974522, 978-1285737232
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



