Question
Which one below is not true for Frenkel and Schottky defects in ionic solids? * . (4 Puan) O Frenkel defect involves a cation-vacancy

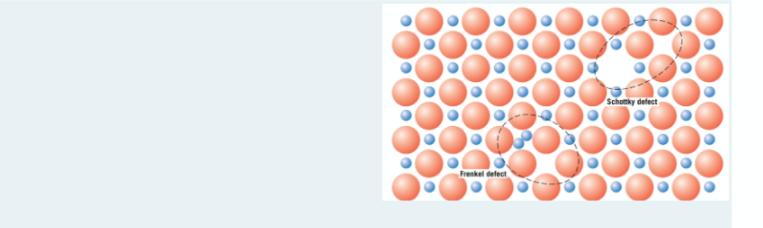
Which one below is not true for Frenkel and Schottky defects in ionic solids? * . (4 Puan) O Frenkel defect involves a cation-vacancy and a cation-interstitial pair. When Frenkel defect occurs, there is no change in charge because the cation maintains the same positive charge as an interstitial. When Schottky defect occurs, the charge neutrality of the crystal is not maintained because it involves both anions and cations. O Schottky defect involves a cation vacancy and an anion vacancy pair. Schutky defect Frenkel defect
Step by Step Solution
3.45 Rating (168 Votes )
There are 3 Steps involved in it
Step: 1
Answer The correct option is C Explanation Option 3rd is answer Explanation Frenkel ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry
Authors: Raymond Chang
10th edition
77274318, 978-0077274313
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



