Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Which one of the metal atoms whose electronic structure given below forms a nitrate of the type M(NO3)2? 37 A. 2.8.0 B. 2.8.1 C.

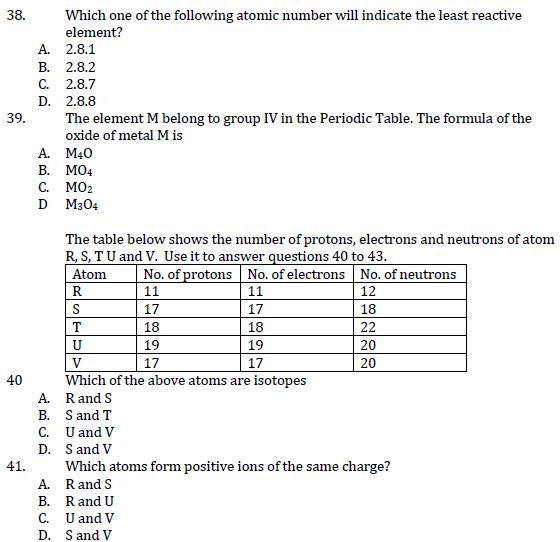
Which one of the metal atoms whose electronic structure given below forms a nitrate of the type M(NO3)2? 37 A. 2.8.0 B. 2.8.1 C. 2.8.2 D. 2.8.3 38. Which one of the following atomic number will indicate the least reactive element? A. 2.8.1 B. 2.8.2 C. 2.8.7 D. 2.8.8 The element M belong to group IV in the Periodic Table. The formula of the oxide of metal M is 39. A. B. MO4 M40 40 40 C. MO2 D M304 The table below shows the number of protons, electrons and neutrons of atom R, S, TU and V. Use it to answer questions 40 to 43. Atom No. of protons No. of electrons No. of neutrons R 11 11 S 17 17 T 18 18 U 19 19 V 17 17 Which of the above atoms are isotopes A. R and S B. S and T C. U and V 12 18 22 20 20 D. S and V Which atoms form positive ions of the same charge? 41. A. R and S B. R and U C. U and V D. S and V
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The electronic structure of MNO32 indicates that the metal atom has a 2 charge since each nitrate ion NO3 carries a 1 charge To determine the electron...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


