Question
Which statement best describes the First Law of Thermodynamics? O O HQ5.24 O A D The change in internal energy of a system is
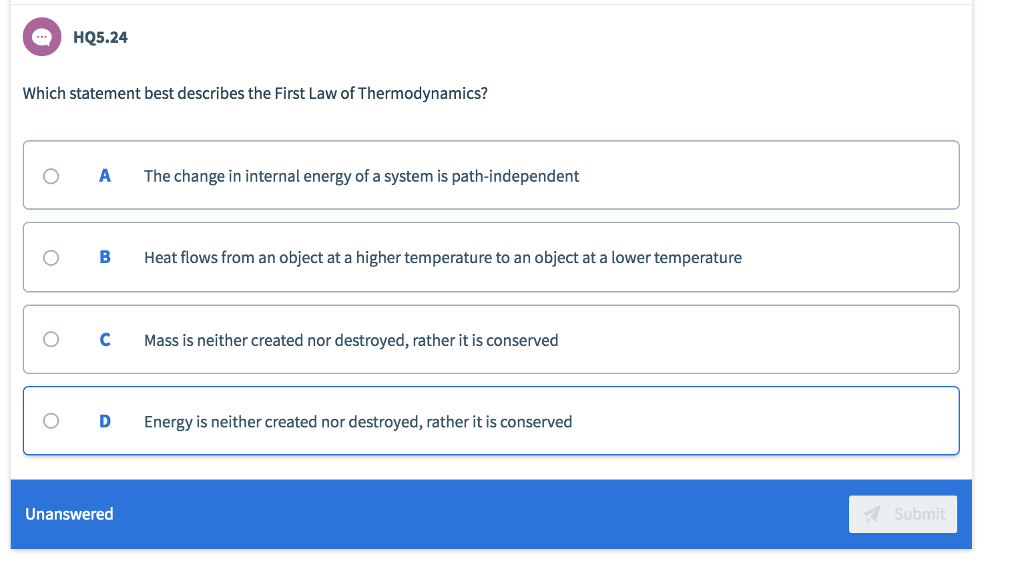
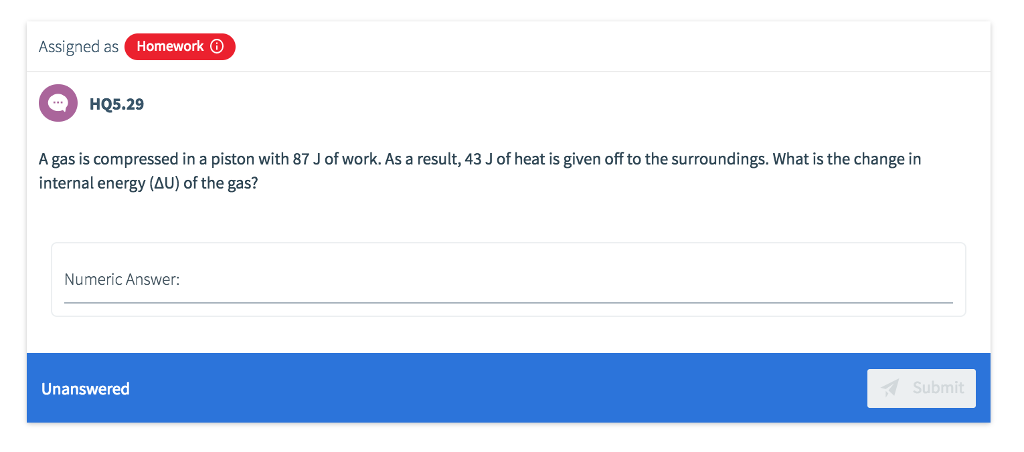
Which statement best describes the First Law of Thermodynamics? O O HQ5.24 O A D The change in internal energy of a system is path-independent Mass is neither created nor destroyed, rather it is conserved Unanswered Heat flows from an object at a higher temperature to an object at a lower temperature Energy is neither created nor destroyed, rather it is conserved Submit Assigned as Homework HQ5.29 A gas is compressed in a piston with 87 J of work. As a result, 43 J of heat is given off to the surroundings. What is the change in internal energy (AU) of the gas? Numeric Answer: Unanswered Submit
Step by Step Solution
3.34 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
1 Answer AThe change in internal energy of a system is pat...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Cambridge International AS And A Level Physics Coursebook
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
3rd Edition
1108859038, 978-1108859035
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



