Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Write a net ionic equation to show that nitric acid, HNO3, behaves as an acid in water. Submit Answer + HO(1) Retry Entire Group
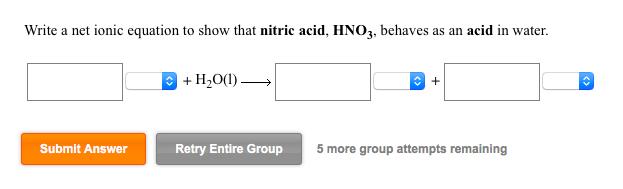
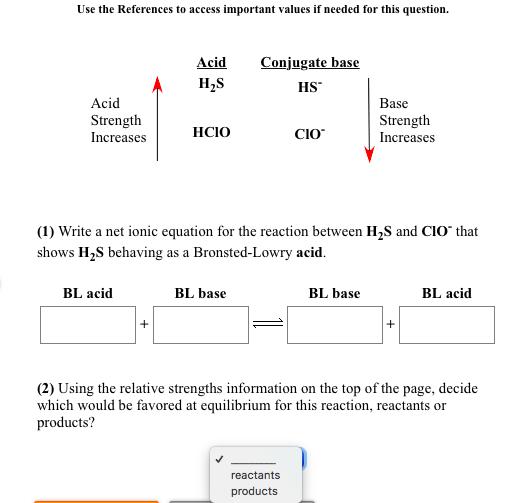
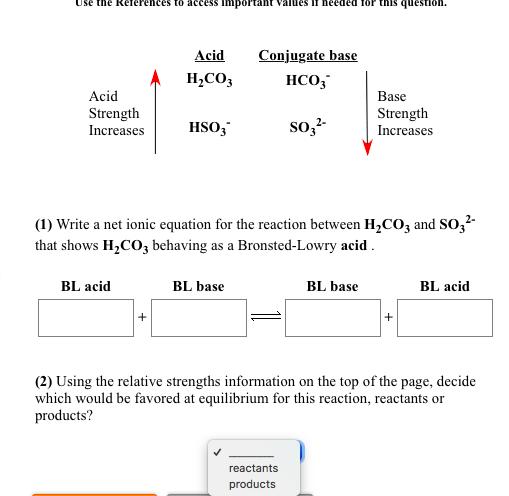

Write a net ionic equation to show that nitric acid, HNO3, behaves as an acid in water. Submit Answer + HO(1) Retry Entire Group + 5 more group attempts remaining Use the References to access important values if needed for this question. Acid Strength Increases BL acid Acid HS + HCIO Conjugate base HS (1) Write a net ionic equation for the reaction between HS and CIO that shows HS behaving as a Bronsted-Lowry acid. BL base CIO reactants products Base Strength Increases BL base + BL acid (2) Using the relative strengths information on the top of the page, decide which would be favored at equilibrium for this reaction, reactants or products? Use the References to access important Acid Strength Increases Acid HCO3 BL acid HSO3* BL base If needed for this question Conjugate base HCO3 SO3- (1) Write a net ionic equation for the reaction between HCO3 and SO3- that shows HCO3 behaving as a Bronsted-Lowry acid. reactants products Base Strength Increases BL base + BL acid (2) Using the relative strengths information on the top of the page, decide which would be favored at equilibrium for this reaction, reactants or products? Write a net ionic equation to show that hydrofluoric acid, HF, behaves as an acid in water. Submit Answer + HO Retry Entire Group + 5 more group attempts remaining
Step by Step Solution
★★★★★
3.58 Rating (169 Votes )
There are 3 Steps involved in it
Step: 1
Bransted Lowery acid BL acid is any species that can donate Htion to another mol...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


