Question
The following reaction begins when 0.56 mol of CO and 0.64 mol of H2O are added to a 2.00 L reaction vessel at a
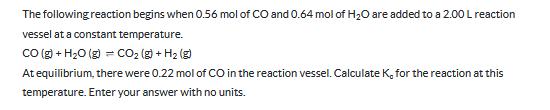
The following reaction begins when 0.56 mol of CO and 0.64 mol of H2O are added to a 2.00 L reaction vessel at a constant temperature. CO(g) + H2O (g) = CO2(g) + H2(g) At equilibrium, there were 0.22 mol of CO in the reaction vessel. Calculate K, for the reaction at this temperature. Enter your answer with no units.
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1
The balanced chemical equation for the given reaction is COg H2Og CO2g H2g The reaction quotient Qc ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Design And Analysis Of Experiments
Authors: Douglas C., Montgomery
5th Edition
978-0471316497, 0471316490
Students also viewed these Programming questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



