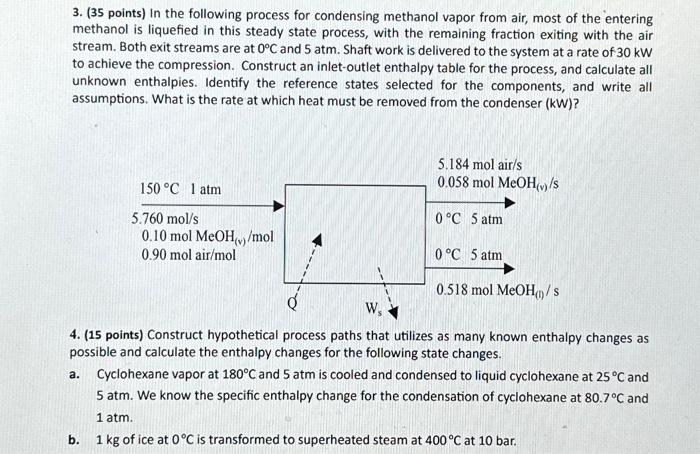
Write the answers for each question on paper. Show all derivations, calculations, graphs, and units. Scan the solution, convert to PDF, and submit on Moodle before the deadline. 1. (25 points) Three streams with the following properties are mixed: Feed stream 1: Superheated steam at 540C and 1.0 bar with the mass flow rate of m1=200kg/h. Feed stream 2: Superheated steam at 710C and 1.0 bar. Feed stream 3: Saturated steam at 1.0 bar with the mass flow rate of m3=400kg/h. The mixing unit operates adiabatically. The exiting stream of the mixing process is a superheated steam at 1.0 bar and its specific enthalpy is H4=3650kJ/kg. a. Draw and label the flowchart of the process. b. Calculate the mass flow rates of the second feed stream (m2) and the product stream (m4) in kg/h. c. Calculate the temperature (Ti) of the superheated steam emerging from the process. d. Determine the temperature (T3) of the third stream. e. Determine the specific volume (V^2) of the second stream in m3/kg. 2. (25 points) Water flows through the following system. Pressures and velocities of water at point 1 and at point 2 are as follows. Calculate Ws in kW, assuming the friction losses are negligible. Mass flow rate of the water is 1000kg/s.P1=300kPa,P2=150kPa,u1=3m/s, and u2=8m/s. 3. ( 35 points) In the following process for condensing methanol vapor from air, most of the entering methanol is liquefied in this steady state process, with the remaining fraction exiting with the air stream. Both exit streams are at 0C and 5atm. Shaft work is delivered to the system at a rate of 30kW to achieve the compression. Construct an inlet-outlet enthalpy table for the process, and calculate all unknown enthalpies. Identify the reference states selected for the components, and write all assumptions. What is the rate at which heat must be removed from the condenser (kW)? 4. (15 points) Construct hypothetical process paths that utilizes as many known enthalpy changes as possible and calculate the enthalpy changes for the following state changes. a. Cyclohexane vapor at 180C and 5atm is cooled and condensed to liquid cyclohexane at 25C and 5atm. We know the specific enthalpy change for the condensation of cyclohexane at 80.7C and 1atm. b. 1kg of ice at 0C is transformed to superheated steam at 400C at 10 bar