Question
Write the full chemical reaction for the extraction of elemental sodium from sodium chloride. Be sure to balance the equation and include the correct
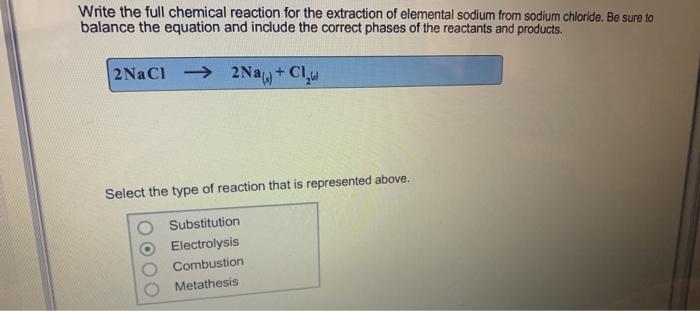
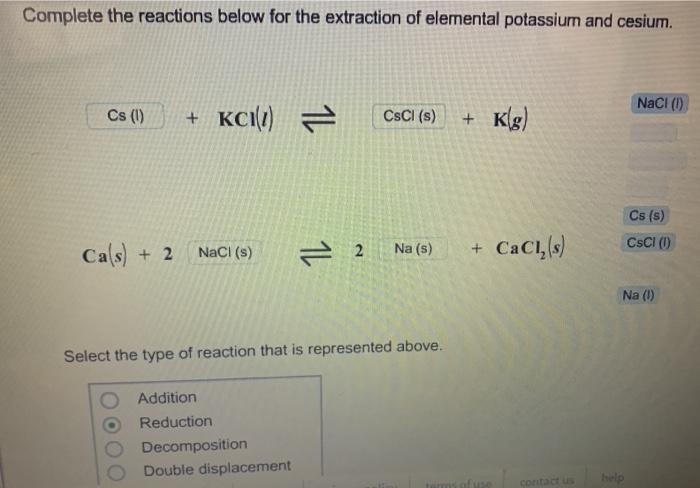
Write the full chemical reaction for the extraction of elemental sodium from sodium chloride. Be sure to balance the equation and include the correct phases of the reactants and products. 2NaCl2Na+ Cl Select the type of reaction that is represented above. Substitution Electrolysis Combustion Metathesis Complete the reactions below for the extraction of elemental potassium and cesium. Cs (1) + KCI(1) = Ca(s) + 2 NaCl (s) 14 Addition Reduction. Decomposition Double displacement CsCl (s) Na (s) Select the type of reaction that is represented above. terms + Kg) +CaCl (s) contact us NaCl (1) help Cs (s) CsCl (1) Na (1)
Step by Step Solution
3.43 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
Ari 2 Nace Ans 11 In this reaction cathode at at anod...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



