Question
Write the half-reactions as they occur at each electrode and the net cell reaction for this electrochemical cell containing indium and cadmium: In(s)|In (aq)||Cd?*(aq)|Cd(s)
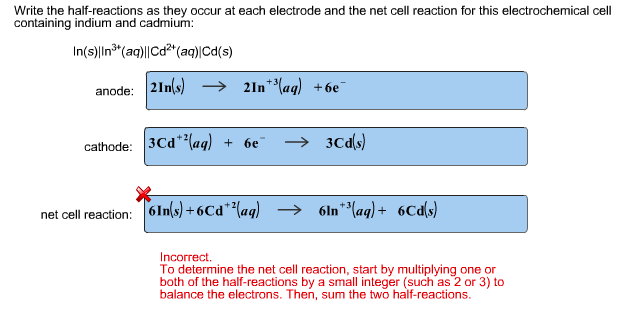
Write the half-reactions as they occur at each electrode and the net cell reaction for this electrochemical cell containing indium and cadmium: In(s)|In (aq)||Cd?*(aq)|Cd(s) anode: 2In(s) 2ln**(aq) +6e cathode: 3Cd"?(aq) + 6e 3Ca(s) net cell reaction: 6In(s) + 6Cd*(aq) 6ln**(aq) + 6Cd(s) Incorrect. To determine the net cell reaction, start by multiplying one or both of the half-reactions by a small integer (such as 2 or 3) to balance the electrons. Then, sum the two half-reactions.
Step by Step Solution
3.42 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
The given cell representation is In s In aqCd aq Cds In th...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Peter Atkins
7th Edition
978-0716735397, 716735393, 978-0716743880
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



