Answered step by step
Verified Expert Solution
Question
1 Approved Answer
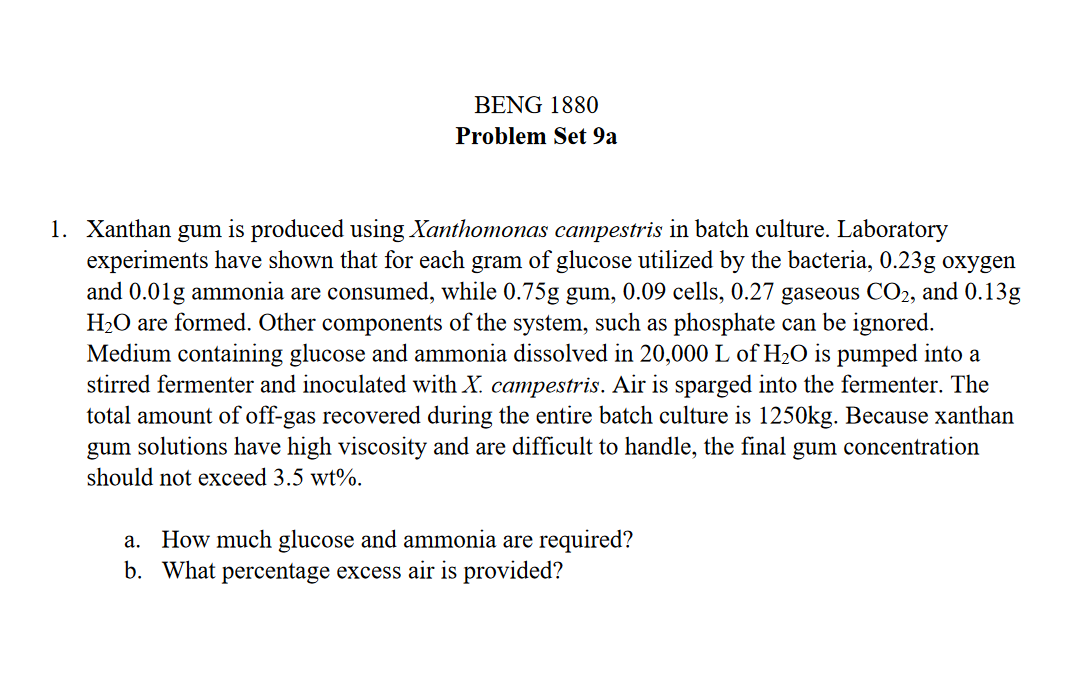
Xanthan gum is produced using Xanthomonas campestris in batch culture. Laboratory experiments have shown that for each gram of glucose utilized by the bacteria, 0
Xanthan gum is produced using Xanthomonas campestris in batch culture. Laboratory
experiments have shown that for each gram of glucose utilized by the bacteria, oxygen
and ammonia are consumed, while gum, cells, gaseous and
are formed. Other components of the system, such as phosphate can be ignored.
Medium containing glucose and ammonia dissolved in of is pumped into a
stirred fermenter and inoculated with campestris. Air is sparged into the fermenter. The
total amount of offgas recovered during the entire batch culture is Because xanthan
gum solutions have high viscosity and are difficult to handle, the final gum concentration
should not exceed
a How much glucose and ammonia are required?
b What percentage excess air is provided?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


