Question
Sulfur tetrafluoride gas is collected at 34.0 C in an evacuated flask with a measured volume of 5.0 L. When all the gas has
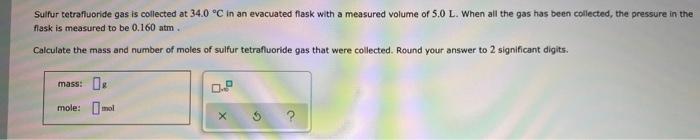
Sulfur tetrafluoride gas is collected at 34.0 C in an evacuated flask with a measured volume of 5.0 L. When all the gas has been collected, the pressure in the flask is measured to be 0.160 atm. Calculate the mass and number of moles of sulfur tetrafluoride gas that were collected. Round your answer to 2 significant digits. mass: mole: mol 0.P
Step by Step Solution
3.45 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
2 NoNz 8 2 Na 8 3N gl Sfy sulfur tetrafluoride Molar mass 108 gmol ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Contemporary Auditing
Authors: Michael C. Knapp
8th edition
978-0538466790, 538466790, 978-1285066608
Students also viewed these General Management questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



