Answered step by step
Verified Expert Solution
Question
1 Approved Answer
You are given the following Gibbs free energy: aT5/2 G = -kBTNln P Where kg is the Boltzmann constant, N is the number of
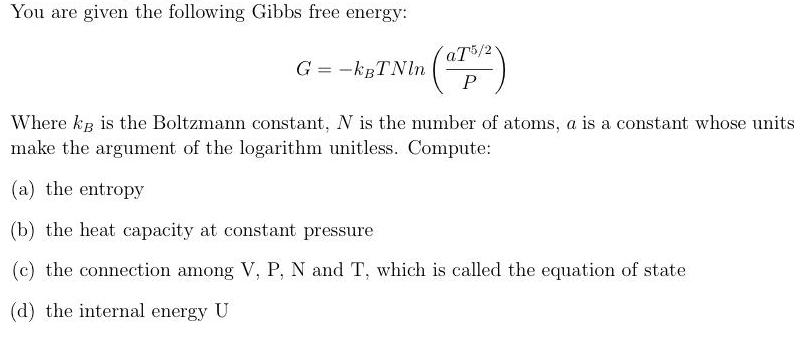
You are given the following Gibbs free energy: aT5/2 G = -kBTNln P Where kg is the Boltzmann constant, N is the number of atoms, a is a constant whose units make the argument of the logarithm unitless. Compute: (a) the entropy (b) the heat capacity at constant pressure (c) the connection among V, P, N and T, which is called the equation of state (d) the internal energy U
Step by Step Solution
★★★★★
3.40 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
aThe relation between the entropy and the Gibbs free energ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


