Answered step by step
Verified Expert Solution
Question
1 Approved Answer
you can solve all if you want i just dont get part e Solve all problems showing all necessary steps and assumptions (Table B.2 in
you can solve all if you want i just dont get part e 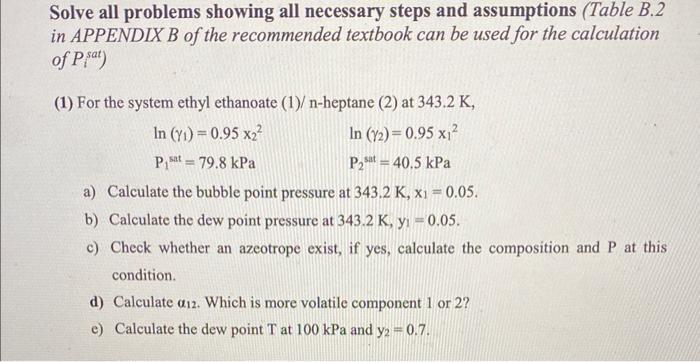
Solve all problems showing all necessary steps and assumptions (Table B.2 in APPENDIX B of the recommended textbook can be used for the calculation of Pisat) (1) For the system ethyl ethanoate (1)-heptane (2) at 343.2K, ln(1)=0.95x22P1sat=79.8kPaln(2)=0.95x12P2satt=40.5kPa a) Calculate the bubble point pressure at 343.2K,x1=0.05. b) Calculate the dew point pressure at 343.2K,y1=0.05. c) Check whether an azeotrope exist, if yes, calculate the composition and P at this condition. d) Calculate 12. Which is more volatile component 1 or 2 ? e) Calculate the dew point T at 100kPa and y2=0.7 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


