Answered step by step
Verified Expert Solution
Question
1 Approved Answer
You don't have to do the actual calculations, but could you answer a & b? One hundred pounds of 50:50 (mol) CO2:C2H6 are loaded into
You don't have to do the actual calculations, but could you answer a & b? 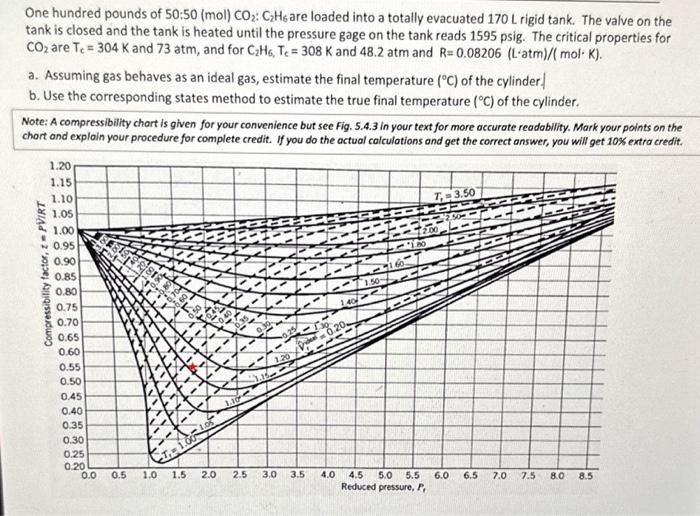
One hundred pounds of 50:50 (mol) CO2:C2H6 are loaded into a totally evacuated 170L rigid tank. The valve on the tank is closed and the tank is heated until the pressure gage on the tank reads 1595 psig. The critical properties for CO2 are Tc=304K and 73atm, and for C2H6Tc=308K and 48.2atm and R=0.08206(Latm)/(molK). a. Assuming gas behaves as an ideal gas, estimate the final temperature (C) of the cylinder. b. Use the corresponding states method to estimate the true final temperature (C) of the cylinder. Note: A compressibility chart is given for your convenience but see Fig. 5.4.3 in your text for more accurate readability. Mark your points on the chart and explain your procedure for complete credit. If you do the actual calculations and get the correct answer, you will get 10% extra credit 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


