Answered step by step
Verified Expert Solution
Question
1 Approved Answer
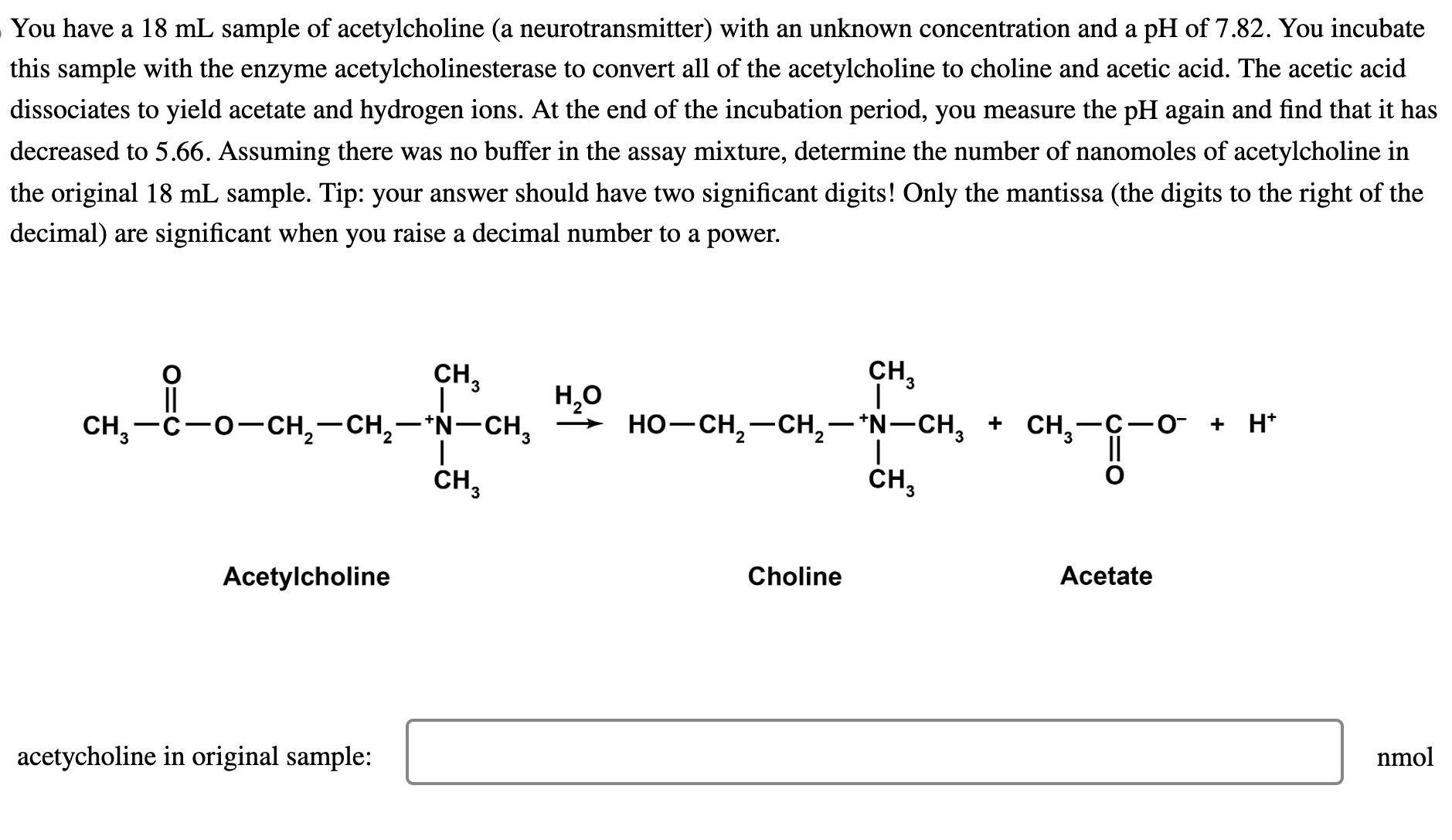
You have a 1 8 m L sample of acetylcholine ( a neurotransmitter ) with an unknown concentration and a pH of 7 . 8
You have a sample of acetylcholine a neurotransmitter with an unknown concentration and a pH of You incubate
this sample with the enzyme acetylcholinesterase to convert all of the acetylcholine to choline and acetic acid. The acetic acid
dissociates to yield acetate and hydrogen ions. At the end of the incubation period, you measure the again and find that it has
decreased to Assuming there was no buffer in the assay mixture, determine the number of nanomoles of acetylcholine in
the original sample. Tip: your answer should have two significant digits! Only the mantissa the digits to the right of the
decimal are significant when you raise a decimal number to a power.
Acetylcholine
Choline
Acetate
acetycholine in original sample:
nmol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


