Answered step by step
Verified Expert Solution
Question
1 Approved Answer
equation, the dependence of the vapor pressure of a pure dln (p/p) AvapHm. Therefore, a plot of In (p/p) . d(1/T) R According to
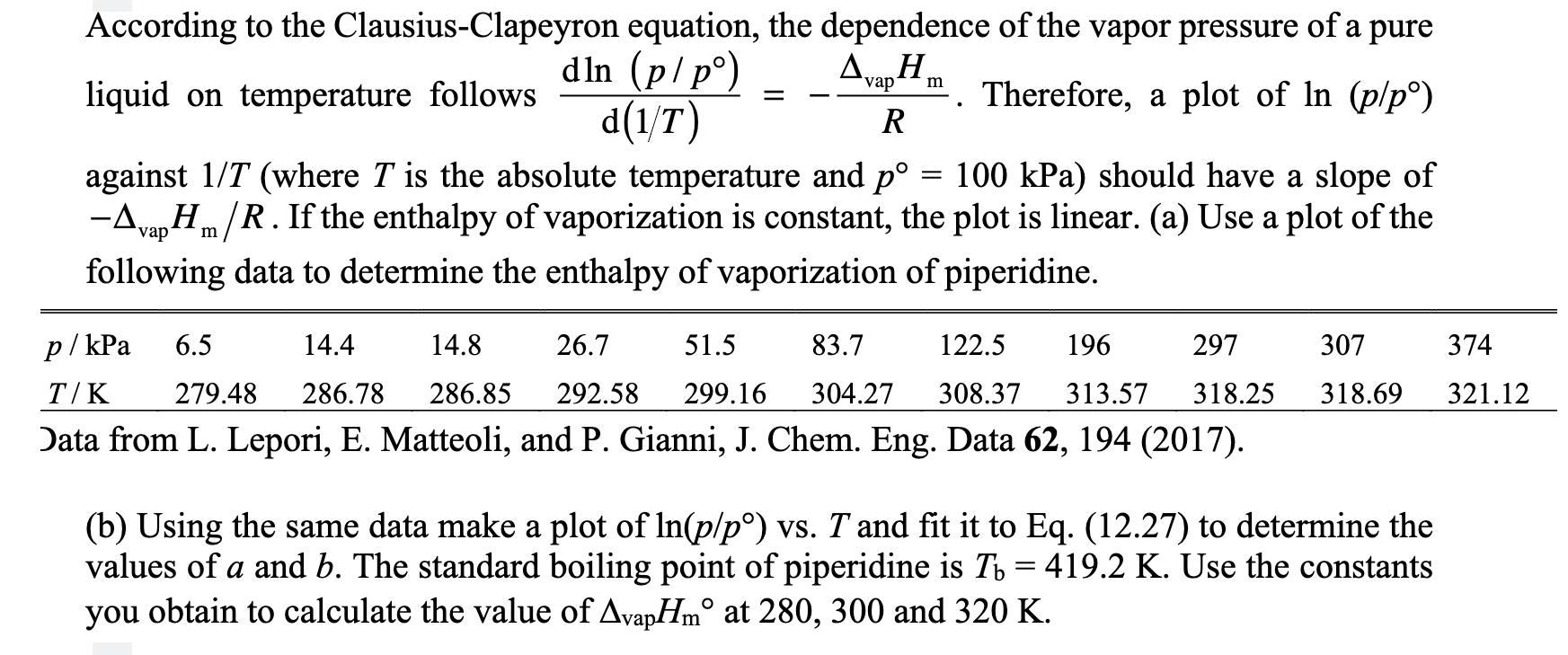
equation, the dependence of the vapor pressure of a pure dln (p/p) AvapHm. Therefore, a plot of In (p/p) . d(1/T) R According to the Clausius-Clapeyron liquid on temperature follows . against 1/T (where T is the absolute temperature and p 100 kPa) should have a slope of -Avap Hm/R. If the enthalpy of vaporization is constant, the plot is linear. (a) Use a plot of the following data to determine the enthalpy of vaporization of piperidine. = p/kPa 6.5 14.4 14.8 26.7 51.5 83.7 122.5 196 297 307 374 T/K 279.48 286.78 286.85 292.58 299.16 304.27 308.37 313.57 318.25 318.69 321.12 Data from L. Lepori, E. Matteoli, and P. Gianni, J. Chem. Eng. Data 62, 194 (2017). (b) Using the same data make a plot of ln(p/p) vs. T and fit it to Eq. (12.27) to determine the values of a and b. The standard boiling point of piperidine is Tb = 419.2 K. Use the constants you obtain to calculate the value of AvapHm at 280, 300 and 320 K.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


