Answered step by step
Verified Expert Solution
Question
1 Approved Answer
You have been given a jar of red fruit punch and been asked to determine the concentration of red dye in the fruit punch. You
You have been given a jar of red fruit punch and been asked to determine the concentration of red dye in the fruit punch. You obtain a stock solution of this red dye and it has a concentration of 0.00113 M (in the lab room, the stock solution is found in the hood). You take 10.0 mL of this stock solution and dilute it to 100.0 mL. You call this your standard solution. Using a colorimeter, you determine that the red dye standard absorbs green light (the complementary color) with an absorbance value of 0.65.
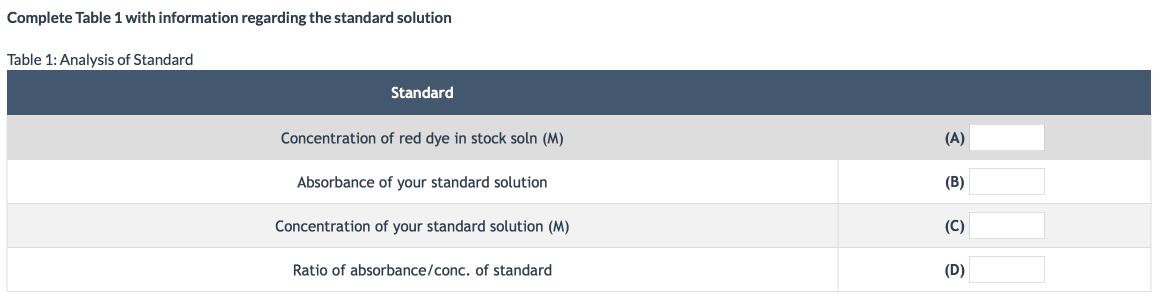
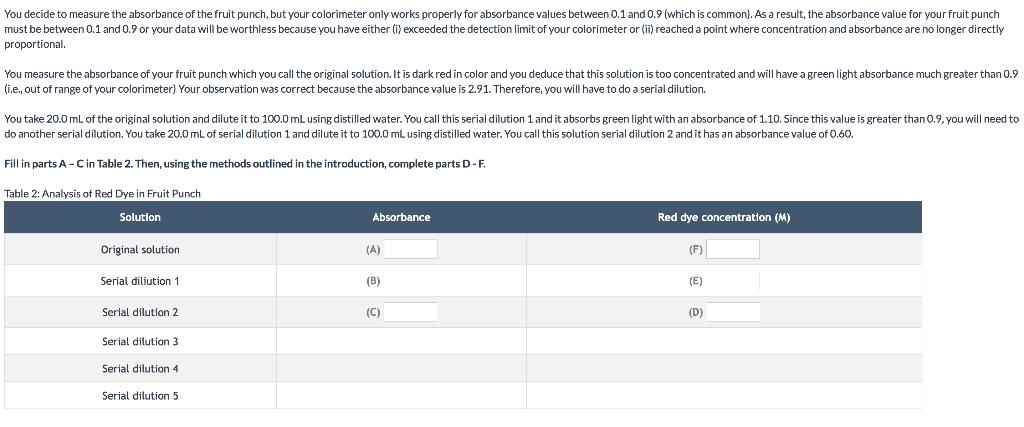
Complete Table 1 with information regarding the standard solution Table 1: Analysis of Standard Standard Concentration of red dye in stock soln (M) (A) Absorbance of your standard solution (B) Concentration of your standard solution (M) (C) Ratio of absorbance/conc. of standard (D)
Step by Step Solution
★★★★★
3.44 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
The concentration of the red dye is the solution stock 000330 ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


