Answered step by step
Verified Expert Solution
Question
1 Approved Answer
You have isolated a pentapeptide composed of four glycine resi- dues and one lysine residue that resides at the Cterminus of the peptide. Using the
You have isolated a pentapeptide composed of four glycine resi- dues and one lysine residue that resides at the C‐terminus of the peptide. Using the information provided in the legend of Figure 2.27, if the pK of the side chain of lysine is 10 and the pK of the terminal carboxyl group is 4, what is the structure of the peptide at pH 7? At pH 12?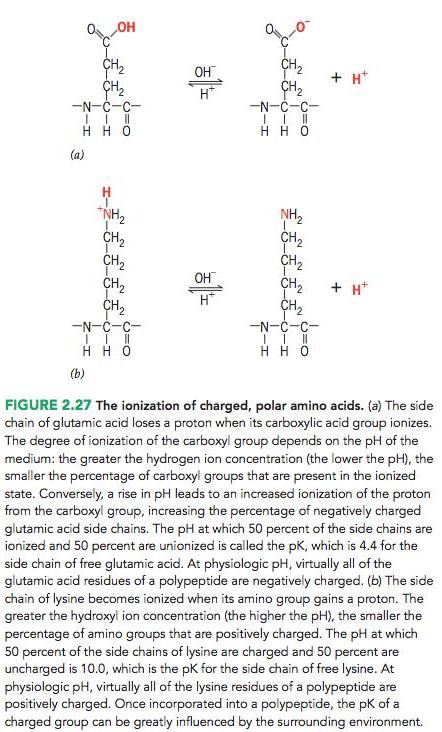
CH2 CH2 -N-C-C- OH + H* H* -N-C-C- H H (a) *NH2 CH2 CH2 CH2 CH2 NH2 CH2 CH2 CH2 OH + H* H* -N-C-C- -N-C-C- H H 0 (b) FIGURE 2.27 The ionization of charged, polar amino acids. (a) The side chain of glutamic acid loses a proton when its carboxylic acid group ionizes. The degree of ionization of the carboxyl group depends on the pH of the medium: the greater the hydrogen ion concentration (the lower the pH), the smaller the percentage of carboxyl groups that are present in the ionized state. Conversely, a rise in pH leads to an increased ionization of the proton from the carboxyl group, increasing the percentage of negatively charged glutamic acid side chains. The pH at which 50 percent of the side chains are ionized and 50 percent are unionized is called the pK, which is 4.4 for the side chain of free glutamic acid. At physiologic pH, virtually all of the glutamic acid residues of a polypeptide are negatively charged. (b) The side chain of lysine becomes ionized when its amino group gains a proton. The greater the hydroxyl ion concentration (the higher the pH), the smaller the percentage of amino groups that are positively charged. The pH at which 50 percent of the side chains of lysine are charged and 50 percent are uncharged is 10.0, which is the pk for the side chain of free lysine. At physiologic pH, virtually all of the lysine residues of a polypeptide are positively charged. Once incorporated into a polypeptide, the pK of a charged group can be greatly influenced by the surrounding environment.
Step by Step Solution
★★★★★
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


