Question
You must present detailed calculations on preparing the solutions listed below, a. 25 mL of 0.1 M Cu(NO3)2 solution in deionised water b. 25
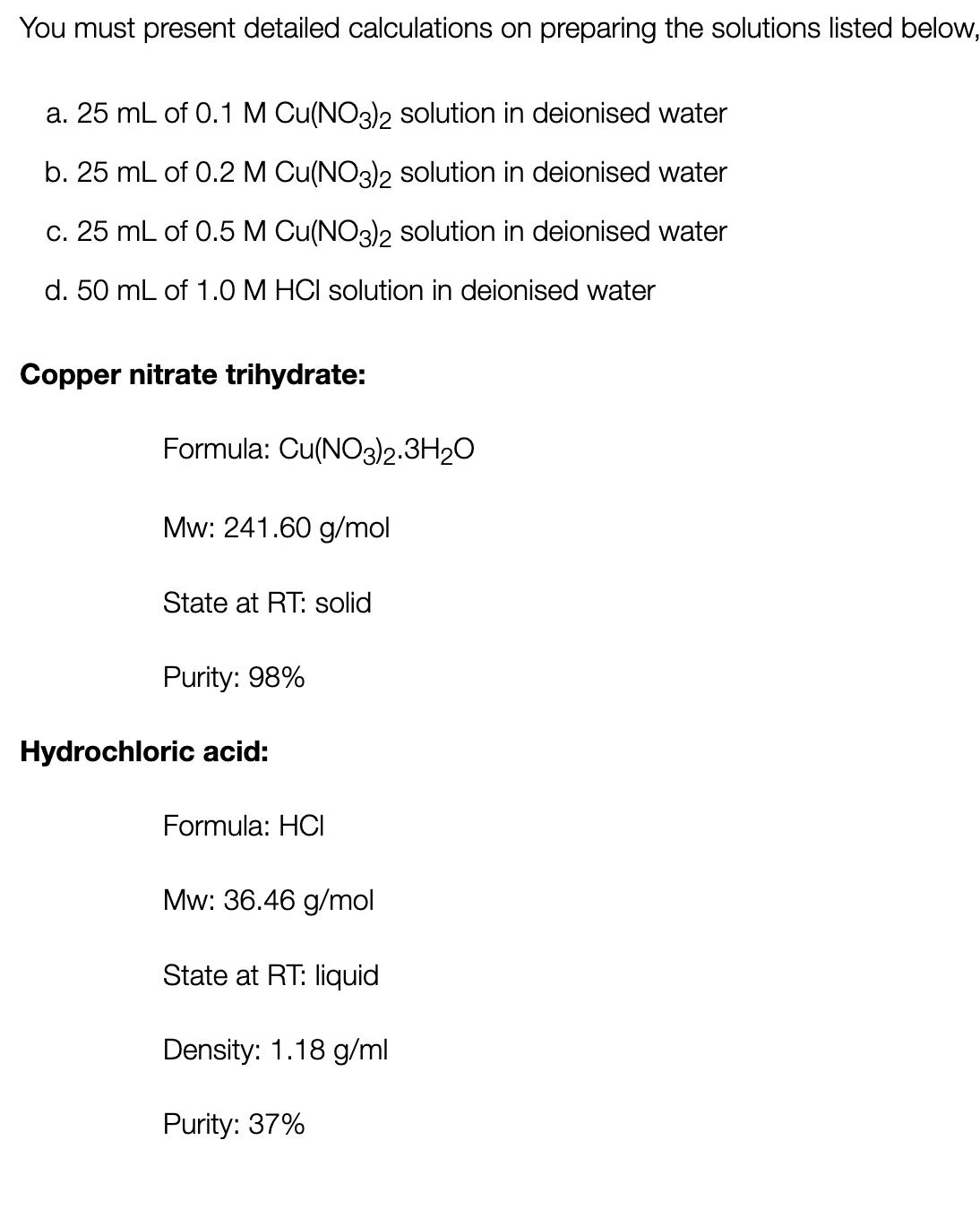
You must present detailed calculations on preparing the solutions listed below, a. 25 mL of 0.1 M Cu(NO3)2 solution in deionised water b. 25 mL of 0.2 M Cu(NO3)2 solution in deionised water c. 25 mL of 0.5 M Cu(NO3)2 solution in deionised water d. 50 mL of 1.0 M HCI solution in deionised water Copper nitrate trihydrate: Formula: Cu(NO3)2.3H2O Mw: 241.60 g/mol State at RT: solid Purity: 98% Hydrochloric acid: Formula: HCI Mw: 36.46 g/mol State at RT: liquid Density: 1.18 g/ml Purity: 37%
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Nature Of Mathematics
Authors: Karl J. Smith
13th Edition
1133947255, 978-1133947257
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



